Featured new articles related to intranasal drug delivery
January-December 2020:
________________________________________________________________
Bouida, W., K. Bel Haj Ali, et al. (2020). "Effect on Opioids
Requirement of Early Administration of Intranasal Ketamine for Acute
Traumatic Pain." Clin J Pain
36(6): 458-462.
Abstract:
OBJECTIVES: To evaluate the efficacy and safety of early administration of low-dose intranasal ketamine on reducing the need for opioid and nonopioid analgesic agents in emergency department (ED) patients with acute moderate to severe acute limbs' trauma pain. PATIENTS AND METHODS: This is a double-blind, randomized, prospective, controlled study conducted in the ED. The included patients were randomly assigned to intranasal pulverization of ketamine or placebo. Protocol treatment was given at the triage. The primary outcome is the need for opioids during ED stay. Secondary outcome included the requirement of nonopioid analgesic agents and the percentage of patients discharged from the ED with a visual analog scale (VAS) <30. A combined outcome score including the 3 outcome items was constructed. RESULTS: The authors included 1102 patients, 550 patients in the placebo group, and 552 in the intranasal ketamine group. The groups were similar regarding demographics, clinical characteristics, and baseline VAS. The need for opioids was decreased in the intranasal ketamine group compared with the placebo group (17.2% vs. 26.5%; P<0.001). The need for nonopioid analgesics was significantly lower in the intranasal ketamine group compared with the placebo group (31.1% vs. 39.6%; P=0.003). The percentage of patients discharged with a VAS score <30 was significantly higher in the intranasal ketamine group (P<0.001). The mean combined outcome score was 0.97 in the placebo group and 0.67 in the intranasal ketamine group (P<0.001). CONCLUSION: Intranasal ketamine administered early in the triage was associated with a decrease in opioids and nonopioid analgesics need in patients with acute limb trauma-related pain.
Web site Editorial comments:
Here is another nasal ketamine for emergency department
pain control study with a slight twist – triage delivery
to speed onset of action and influence further analgesic use during the
ED stay.
These authors conducted a double blind RCT that looked at the impact
on opiate use in the ED in patients with acute limb injury who were
randomized to IN ketamine vs placebo delivered at triage. They found
that triage delivered IN ketamine led to less opiate and non-opiate
analgesic use
and better overall pain scores at discharge.
This study lends credence to the idea of combination therapy for
nasal analgesics if you wish to reduce overall opiate dose (both can be
give IN). It also would allow institutions that are reluctant to give an
opiate at triage another option for treating patients with severe pain –
nasal ketamine.
It is a potentially useful concept to add to your
armamentarium of treatment options in the ED.
Pubmed link:
________________________________________________________________
Crawshaw, A. A. and H. R. Cock (2020). "Medical management of status epilepticus: Emergency room to intensive care unit." Seizure 75: 145-152.
Abstract: In convulsive status epilepticus (SE), achieving seizure control within the first 1-2hours after onset is a significant determinant of outcome. Treatment is also more likely to work and be cost effective the earlier it is given. Initial first aid measures should be accompanied by establishing intravenous access if possible and administering thiamine and glucose if required. Calling for help will support efficient management, and also the potential for video-recording the events. This can be done as a best interests investigation to inform later management, provided adequate steps to protect data are taken. There is high quality evidence supporting the use of benzodiazepines for initial treatment. Midazolam (buccal, intranasal or intramuscular) has the most evidence where there is no intravenous access, with the practical advantages of administration outweighing the slightly slower onset of action. Either lorazepam or diazepam are suitable IV agents. Speed of administration and adequate initial dosing are probably more important than choice of drug. Although only phenytoin (and its prodrug fosphenytoin) and phenobarbitone are licensed for established SE, a now considerable body of evidence and international consensus supports the utility of both levetiracetam and valproate as options in established status. Both also have the advantage of being well tolerated as maintenance treatment, and possibly a lower risk of serious adverse events. Two adequately powered randomized open studies in children have recently reported, supporting the use of levetiracetam as an alterantive to phenytoin. The results of a large double blind study also including valproate are also imminent, and together likely to change practice in benzodiazepine-resistant SE.
Web site Editorial comments:
This is a nice review of the treatment of status epilepticus in a
reputable journal that you can access for free (click here for the PDF).
These authors have put transmucosal benzodiazepines into their status
epilepticus algorithm as the first line therapy in patients without an
IV stating “Speed of administration and adequate initial dosing are
probably more important than the choice of drug.”
(Free access on internet – click here for PDF)
________________________________________________________________
Domany, Y., J. Lord, et al. (2020). "Intranasal Ketamine for Alleviation of
Acute Suicidal Ideation. An Emergency Department, Trans-Diagnostic
Approach: Randomized, Double-Blind, Placebo-Controlled, Proof-of-Concept
Trial " SSRN(http://dx.doi.org/10.2139/ssrn.3367057).
Abstract:
Background: suicidal patients are often presented to
the Emergency Department, where specific treatment is lacking. Ketamine,
a rapidly acting antidepressant with anti-suicidal properties might
offer relief.
Methods: thirty eligible participants who suffered
acute suicidal ideation and required hospitalization were randomized to
intranasal ketamine 40mg or placebo, between August 2016 to April 2018.
Safety and efficacy evaluations were scheduled for two and four hours,
on days 1, 3, 7, and 21 post administration. Primary outcome was
suicidal ideation four hours post administration. Randomization was
carried out by the pharmacist while the rest of the study group was
blinded.
Outcomes: Fifteen subjects were randomised for
ketamine and fifteen for control, all were analyzed for primary and
secondary outcomes. Four hours post administration the mean difference
in suicidal symptoms between the groups, measured by the Montgomery-Åsberg
Depression Rating Scale (MADRS) item of suicidal thoughts, was 1.267
(95% confident interval 0.1-2.43, P<0.05) favoring the ketamine group,
with suicidal ideation remission rates of 80% of the ketamine group
compares with 33% of the controls (p<0.05). The mean difference in
depressive symptoms, measured by MADRS, at the same time was 9.75 (95%
confident interval 0.72-18.79, P<0.05) favoring the ketamine group. The
treatment was safe and well-tolerated.
Interpretation: Intranasal ketamine alleviated
suicidal ideation and improved depressive symptoms four hours post
ketamine administration. We present an innovative paradigm for the
management of suicidal individuals in emergency setting. Future
larger-scale studies are warranted to establish treatment
recommendation.
Web site Editorial comments:
This may be the first time I have featured this topic in this
section of the website. I have been following the idea of IN ketamine
for acute relief of depression for well over a decade. Here is a small
but well designed study that encapsulates the concept. Basically in
patients with acute suicidal ideations or even chronic depression it
appears that a single dose of nasal ketamine often provides them
substantial relief from their symptoms.
I know in our community it has been in use in the
inpatient psych wards for nearly a decade. I confess to not having a
great deal of insight but for anyone who is interested there is a
plethora of data on the topic that you might find insightful.
Free Access on the internet - click here for PDF
________________________________________________________________________________________________________________________________
Huebinger, R. M., H. Q. Zaidi, et al. (2020). "Retrospective Study of
Midazolam Protocol for Prehospital Behavioral Emergencies." West J
Emerg Med 21(3): 677-683.
Abstract: INTRODUCTION: Agitated patients in the prehospital setting pose challenges for both patient care and emergency medical services (EMS) provider safety. Midazolam is frequently used to control agitation in the emergency department setting; however, limited data exist in the prehospital setting. We describe our experience treating patients with midazolam for behavioral emergencies in a large urban EMS system. We hypothesized that using midazolam for acute agitation leads to improved clinical conditions without causing significant clinical deterioration. METHODS: We performed a retrospective review of EMS patient care reports following implementation of a behavioral emergencies protocol in a large urban EMS system from February 2014-June 2016. For acute agitation, paramedics administered midazolam 1 milligram (mg) intravenous (IV), 5 mg intramuscular (IM), or 5 mg intranasal (IN). Results were analyzed using descriptive statistics, Levene's test for assessing variance among study groups, and t-test to evaluate effectiveness based on route. RESULTS: In total, midazolam was administered 294 times to 257 patients. Median age was 30 (interquartile range 24-42) years, and 66.5% were male. Doses administered were 1 mg (7.1%) and 5 mg (92.9%). Routes were IM (52.0%), IN (40.8%), and IV (7.1%). A second dose was administered to 37 patients. In the majority of administrations, midazolam improved the patient's condition (73.5%) with infrequent adverse events (3.4%). There was no significant difference between the effectiveness of IM and IN midazolam (71.0% vs 75.4%; p = 0.24). CONCLUSION: A midazolam protocol for prehospital agitation was associated with reduced agitation and a low rate of adverse events.
Web site Editorial comments:
These authors provide evidence from their EMS system (Chicago EMS) that demonstrate a protocol using either IN or IM midazolam (or occasionally IV) was a safe and effective method of calming agitated EMS patients. Their initial dose was 5 mg IN/IM but they allowed a second dose if needed. The initial dose resulting in improvement in about 80% of cases with substantial improvement in 40%. When another dose was given, there was a similar degree of improvement yet again. There were very few adverse events – primarily nasal and oral discomfort (midazolam has a high pH so it burns on application) but not respiratory compromise.
Personally, having reviewed the intranasal medication literature for
over 20 years and having used IN midazolam in my practice for a similar
time period, it is clear that clinicians tend to be overly conservative
with nasal drug dosing – fearing side effects like respiratory
depression that they have seen when the drug is given intravenously.
With almost NO exceptions, the use of generic IV midazolam (or fentanyl)
via the nasal route will NOT cause any substantial respiratory
depression: It is simply to dilute and is absorbed too slowly (a few
minutes) to acutely depress the respiratory drive. Therefore doses
larger than an IV dose are required to obtain a reliable clinical effect
when administered via the nose. The dose for sedation in children is 0.4
to 0.5 mg/kg. Never the less, after over 30 years of research on this
dose, in children we still routinely see failed sedation studies because
of dosing in the 0.2 mg/kg range.
While I applaud these authors on the data obtained here,
I can vouch from extensive experience that their initial dose is less
than half of what it should be for an adult who is agitated. I would
give 10 mg right up front (2 ml - more volume will just leak out their
nose). However, you need to realize that a 10 mg dose is
less than 0.15 mg/kg in a 70 kg adult so you will rarely see true
sedation at that dose. Titrate up as needed and anxiolysis and some
calming is more likely to occur. Deep sedation is highly unlikely.
Free access on the Internet - click here for PDF
________________________________________________________________
Khalil, W. and N. Raslan (2020). "The effectiveness of topical lidocaine in
relieving pain related to intranasal midazolam sedation: a randomized,
placebo-controlled clinical trial." Quintessence Int
51(2): 162-167.
Abstract: OBJECTIVE: Intranasal midazolam (INM) is an increasingly popular agent for sedation in the emergency department and outside the hospital in physician, imaging, and dental offices, to facilitate diagnostic and minor surgical procedures and avoid the need for an operating room and general anesthesia. The use of INM has been commonly associated with a burning sensation of the nasal mucosa. Despite its significance, this subject has received little adequate research focus. The objective of the current study was to evaluate the effectiveness of topical lidocaine in relieving pain related to INM administration. METHOD AND MATERIALS: This was a blinded, randomized placebo-controlled trial. Sixty-three uncooperative children undergoing dental treatment, aged 4 to 11 years, were randomly assigned to one of three groups to receive the drug nasally via a precalibrated spray as per the following assignments: group A received 0.5 mg/kg midazolam, group B received lidocaine 2% prior to 0.5 mg/kg midazolam, and group C received saline 0.9% (placebo), 0.5 mg/kg. Children were asked to record the degree of pain using the Wong-Baker faces scale. Parental acceptance was also rated. RESULTS: Topical lidocaine prior to INM administration reduced the burning sensation in the nasal mucosa and improved the drug acceptance. The median score of pain was 8, 1, and 4 in groups A, B, and C, respectively. The differences among the three groups were statistically significant (P > .05). The children's acceptance and parents' future acceptance regarding the intranasal drug administration was significantly higher in group B. CONCLUSION: INM administration results in burning sensation in the nasal mucosa with high levels of pain. Using topical lidocaine 2% counteracted the burning sensation and achieved a higher acceptance rate and lower pain scores.
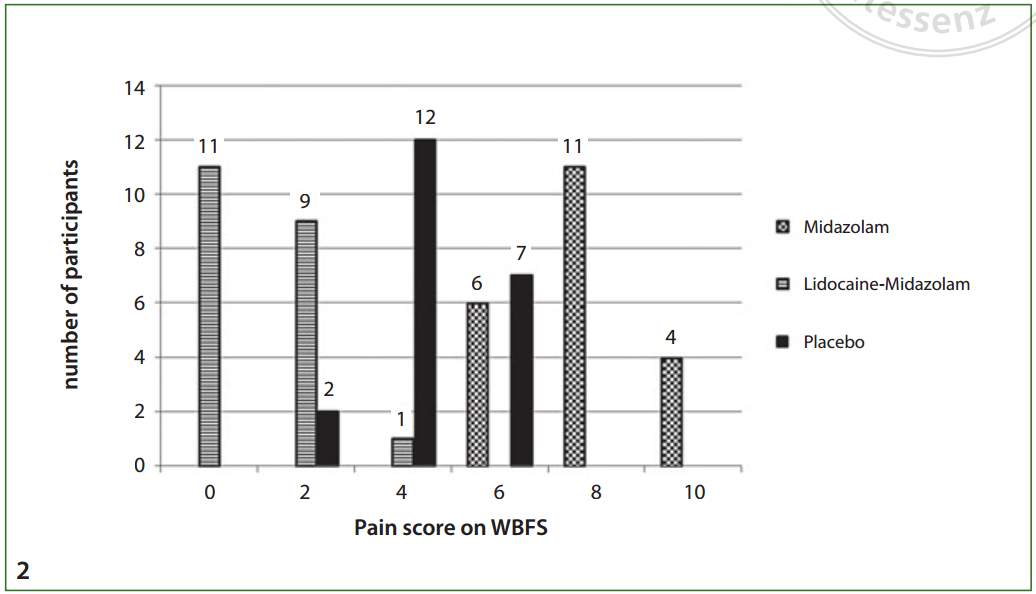
Web site Editorial comments:
Here is one more study to add to the growing list of article
confirming that you should be giving lidocaine either prior to or with
midazolam when
using midazolam for pediatric sedation. Nasal Midazolam burns
and kids cry. Lidocaine blocks that burning and kids don’t cry.
Just do it.
Free access on the Internet - click here for PDF
________________________________________________________________
Sindhur, M., H. Balasubramanian, et al. (2020). "Intranasal fentanyl for
pain management during screening for retinopathy of prematurity in
preterm infants: a randomized controlled trial." J Perinatol
40(6): 881-887.
Abstract:
OBJECTIVE: To study the efficacy of intranasal
fentanyl as an adjunct for pain management during screening for
retinopathy of prematurity (ROP) in preterm infants. STUDY DESIGN: In
this single center, double blinded, randomized controlled trial, preterm
neonates between 30 and 34 weeks postmenstrual age received either
intranasal fentanyl (2 mcg/kg) or intranasal normal saline through a
mucosal atomization device 5 min prior to the first ROP-screening
examination. Both the groups received standard pain relief strategies
(oral sucrose, 0.5% proparacaine eye drops and physical containment).
The primary outcome was premature infant pain profile-revised (PIPP-R)
score during the screening. RESULTS: A total of 111 infants were
enrolled. PIPP-R score during the retinal examination was significantly
lower in the fentanyl group (8.3 versus 11.5, mean difference: 3.2
(2.46-4.06), P < 0.001). There was no significant difference in the
incidence of adverse effects. CONCLUSION: Intranasal fentanyl
significantly reduced the pain associated with retinal examination
without increasing the risk of respiratory depression. Large RCTs are
required to verify the efficacy and safety of intranasal fentanyl for
acute procedural pain in neonates. CLINICAL TRIAL REGISTRATION:
CTRI/2017/12/011016.
Web site Editorial comments:
Here is data showing IN fentanyl is pretty darn safe. These authors
gave it to 111 premature infants (2 mcg/kg – a well established
effective dose) to assist them in assessing retinopathy of prematurity.
They found this treatment significantly reduced pain with NO evidence of
respiratory depression.
________________________________________________________________________________________________________________________________
Title:
Abstract:
Web site Editorial comments:
Pubmed link:
Free access on the Internet - click here for PDF________________________________________________________________
Title:
Abstract:
Web site Editorial comments:
Pubmed link:
________________________________________________________________
Title:
Abstract:
Web site Editorial comments:
Pubmed link:
________________________________________________________________
 Therapeutic
Intranasal Drug Delivery
Therapeutic
Intranasal Drug Delivery