Featured new articles related to intranasal drug delivery
January-December 2019:
Akkan, S., S. K. Corbacioglu, et al. (2019). "Evaluating Effectiveness
of Nasal Compression With Tranexamic Acid Compared With Simple Nasal
Compression and Merocel Packing: A Randomized Controlled Trial." Ann
Emerg Med 74(1): 72-78.
Abstract:
STUDY OBJECTIVE: The primary objective of this study is to compare the
effectiveness of 3 treatment protocols to stop anterior epistaxis:
classic compression, nasal packing, and local application of tranexamic
acid. It also aims to determine the frequency of rebleeding after each
of these protocols. METHODS: This single-center, prospective, randomized
controlled study was conducted with patients who had spontaneous
anterior epistaxis. The study compared the effect of 3 treatment
options, tranexamic acid with compression but without nasal packing,
nasal packing (Merocel), and simple nasal external compression, on the
primary outcome of stopping anterior epistaxis bleeding within 15
minutes. RESULTS: Among the 135 patients enrolled, the median age was 60
years (interquartile range 25% to 75%: 48 to 72 years) and 70 patients
(51.9%) were women. The success rate in the compression with tranexamic
acid group was 91.1% (41 of 45 patients); in the nasal packing group,
93.3% (42 of 45 patients); and in the compression with saline solution
group, 71.1% (32 of 45 patients). There was an overall statistically
significant difference among the 3 treatment groups but no significant
difference in pairwise comparison between the compression with
tranexamic acid and nasal packing groups. In regard to no rebleeding
within 24 hours, the study found rates of 86.7% in the tranexamic acid
group, 74% in the nasal packing group, and 60% in the compression with
saline solution group. CONCLUSION: Applying external compression after
administering tranexamic acid through the nostrils by atomizer stops
bleeding as effectively as anterior nasal packing using Merocel. In
addition, the tranexamic acid approach is superior to Merocel in terms
of decreasing rebleeding rates.
Web site Editorial comments:
This study is a randomized trial comparing three treatment modalities
for epistaxis: Atomized placebo (saline) with compression, Atomized
tranexamic acid with compression and nasal packing with Merocel.
Efficacy at stopping bleeding by 10 minutes was 71%, 91% and 93%
respectively. Rebleeding at 24 hours was 40%, 13% and 26% respectively.
Nasal packing was found to be quite uncomfortable.
Epistaxis can be a messy problem in the ED when traditional packing
methods are used, and it is quite uncomfortable for the patient.
Utilizing an atomizer along with inexpensive medications such as
tranexamic acid and perhaps some oxymetazoline plus external compression
makes the care of these cases much easier and more comfortable. This is
a great new treatment option for epistaxis and I suspect it will be
rapidly adopted given its low cost and demonstrated efficacy for a
sometimes difficult situation.
________________________________________________________________
Dietze, P., M. Jauncey, et al. (2019). "Effect of Intranasal vs
Intramuscular Naloxone on Opioid Overdose: A Randomized Clinical Trial."
JAMA Netw Open 2(11):
e1914977.
Abstract:
Importance: Previous unblinded clinical trials suggested that the
intranasal route of naloxone hydrochloride was inferior to the widely
used intramuscular route for the reversal of opioid overdose. Objective:
To test whether a dose of naloxone administered intranasally is as
effective as the same dose of intramuscularly administered naloxone in
reversing opioid overdose. Design, Setting, and Participants: A
double-blind, double-dummy randomized clinical trial was conducted at
the Uniting Medically Supervised Injecting Centre in Sydney, Australia.
Clients of the center were recruited to participate from February 1,
2012, to January 3, 2017. Eligible clients were aged 18 years or older
with a history of injecting drug use (n = 197). Intention-to-treat
analysis was performed for all participants who received both intranasal
and intramuscular modes of treatment (active or placebo). Interventions:
Clients were randomized to receive 1 of 2 treatments: (1) intranasal
administration of naloxone hydrochloride 800 mug per 1 mL and
intramuscular administration of placebo 1 mL or (2) intramuscular
administration of naloxone hydrochloride 800 mug per 1 mL and intranasal
administration of placebo 1 mL. Main Outcomes and Measures: The primary
outcome measure was the need for a rescue dose of intramuscular naloxone
hydrochloride (800 mug) 10 minutes after the initial treatment.
Secondary outcome measures included time to adequate respiratory rate
greater than or equal to 10 breaths per minute and time to Glasgow Coma
Scale score greater than or equal to 13. Results: A total of 197 clients
(173 [87.8%] male; mean [SD] age, 34.0 [7.82] years) completed the
trial, of whom 93 (47.2%) were randomized to intramuscular naloxone dose
and 104 (52.8%) to intranasal naloxone dose. Clients randomized to
intramuscular naloxone administration were less likely to require a
rescue dose of naloxone compared with clients randomized to intranasal
naloxone administration (8 [8.6%] vs 24 [23.1%]; odds ratio, 0.35; 95%
CI, 0.15-0.66; P = .002). A 65% increase in hazard (hazard ratio, 1.65;
95% CI, 1.21-2.25; P = .002) for time to respiratory rate of at least 10
and an 81% increase in hazard (hazard ratio, 1.81; 95% CI, 1.28-2.56; P
= .001) for time to Glasgow Coma Scale score of at least 13 were
observed for the group receiving intranasal naloxone compared with the
group receiving intramuscular naloxone. No major adverse events were
reported for either group. Conclusions and Relevance: This trial showed
that intranasally administered naloxone in a supervised injecting
facility can reverse opioid overdose but not as efficiently as
intramuscularly administered naloxone can, findings that largely
replicate those of previous unblinded clinical trials. These results
suggest that determining the optimal dose and concentration of
intranasal naloxone to respond to opioid overdose in real-world
conditions is an international priority. Trial Registration:
anzctr.org.au Identifier: ACTRN12611000852954.
Web site Editorial comments:
These authors used the 0.8 mg in 1 ml naloxone formulation and compared
its efficacy when delivered nasally versus intramuscularly. They
randomized 197 patients. They
found the IM dose to work within 10 minutes in 91% of patients (most
studies using IM. IV or IN find maximum efficacy in the 85-95% range so
this is as good as you can generally expect). The 0.8 mg dose nasally
worked in 77% of patients – a statistically and clinically important
reduction in efficacy.
Comments: For 15 years or more I have been asked if it is ok to give the
0.4 mg/ml standard naloxone dose intranasally to treat opiate overdose.
My answer was always to follow the literature – at the time only 2
mg/2ml had been studied so unless they were conducting a research
project I felt using the 0.4 mg dose was risky as it was potentially too
dilute to be effective. Admittedly Kelly et al published their results
using this formulation in 2005 – but they gave 5 vials of the drug up
the nose (5 ml) so surely had runoff, but did achieve a 2 mg dose. They
found 74% of cases aroused compared to 83% using IM naloxone –
statistically the same. Fast forward to 2014 when Sabzghabaee published
their study on 100 patients where they reported 100% awakening with 0.4
mg IN naloxone – a better result than the 60% awakening with IV
naloxone. This seemed too good to be true and not in line with almost
ALL studies of naloxone where a 90% awakening +/- 5% is more typical
(some patients just don’t wake up due to high levels of opiate or co
ingestion of other sedatives). Today's study by Dietze is more in line
with the Kelly data – they used 0.8 mg intranasally and found 77%
arousal with one dose. So generic IV less concentrated naloxone is
fairly effective when given nasally, but not as effective as the same
dose given intramuscularily. If you ONLY have this lower dose and you
cannot give it IM, then certainly if indicated you should use it
nasally. However there are better nasal options including at least two
commercially available IN naloxone products (2 and 4 mg in 0.1 ml
prepackaged with an atomizer) and the original study drug – 2 mg/2 ml
concentration. You will
need to do your due diligence and price investigation to decide, but if
prices are similar the commercially available drugs are easier to use
and probably very slightly more effective in situations where we are
seeing more synthetic opiate (fentanyl etc) overdoses.
Open Access Full Article Link: https://jamanetwork.com/journals/jamanetworkopen/fullarticle/2755306
________________________________________________________________
Pruyn, S., J. Frey, et al. (2019). "Quality Assessment of Expired
Naloxone Products from First-Responders' Supplies." Prehosp Emerg
Care 23(5): 647-653.
Abstract: Objective: Naloxone is an opioid receptor antagonist that reverses life-threatening effects of opioid overdose. Since the 1970s, naloxone products have been developed as injectable solutions, and more recently as nasal sprays. Naloxone products have saved many lives in emergency settings. These products are routinely carried by public safety first-responders including fire fighters (FF), law enforcement officers (LEO), and emergency medical services (EMS). Now, they are also distributed through community access programs to the public. While public safety medications are monitored, those publically distributed are not, so expired products can be possibly found on-hand in an emergency. This study analyzed the quality and stability of expired Naloxone HCl Solutions for Injection, to assess their remaining efficacies and potential risks. Methods: The samples were collected from EMS or law enforcement training supplies and expired returns, with expiration dates ranging from 1990 to 2018. Using standardized techniques, the remaining naloxone was quantified, and the main degradation products, nornaloxone (also known as noroxymorphone) and other possible species, were monitored and quantified systematically. Results: Most tested samples were found containing more than 90% of labeled naloxone, including those stored for nearly 30 years. The naloxone degradation was slow, but generally correlated with storage time length. There was no significant amount of degradation products detected across all samples. Nornaloxone was detected from some older samples, but all less than 1%. Therefore, although it is an opioid agonist, the risk caused by nornaloxone should be low. Conclusion: This quality assessment demonstrates that expired naloxone products may still meet USP standards, even after many years. Further pharmaceutical, clinical, and regulatory investigation should be conducted to confirm our findings, especially for new naloxone products with different formulations and routes of administration. Extending the shelf-life of naloxone products may have important financial and public health consequences in addressing future drug shortages and meeting the needs for this critical drug.
Web site Editorial comments:
These authors sought to determine if expired naloxone vials contained
drug that was still effective. They tested samples of naloxone with
expiration dates ranging from 1990 to 2018. They found that most samples
still contained over 90% naloxone (not is breakdown product) – even
samples from the 1990’s. Though there was a slight degradation
correlating with time they found no significant degradation that would
effect the drugs use. The conclude that “Extending the shelf-life of
naloxone products may have important financial and public health
consequences in addressing future drug shortages and meeting the needs
for this critical drug.”
Naloxone prices have skyrocketed in the last 20 years. (The opposite of
traditional supply/demand economics because our pharmaceutical industry
does not lie within a traditional economic system). One solution, minor
though it is, might be to use expired drug if it is still effective.
Pruyn showed that to be the case implying that if you have stored your
naloxone out of the light and heat it is likely still fine. Test a vial
– if it works – maybe you should use it. I know for a fact (having been
in the industry) that expiration dates in medical supplies are set based
almost entirely on time required to distribute the product to the shelf
with an expiration date of about 6-12 months or so later. Expiration
dates for these non-spoilable product have almost nothing to do with the
actual shelf life of a product. The
medical industry generally does not test to see how LONG their product
will last, they only test to be sure it can last to the date of delivery
plus 6-12 months beyond.
________________________________________________________________
Andolfatto, G., K. Innes, et al. (2019). "Prehospital Analgesia With
Intranasal Ketamine (PAIN-K): A Randomized Double-Blind Trial in
Adults." Ann Emerg Med 74(2):
241-250.
Abstract: STUDY OBJECTIVE: We compare intranasal ketamine with intranasal placebo in providing pain reduction at 30 minutes when added to usual paramedic care with nitrous oxide. METHODS: This was a randomized double-blind study of out-of-hospital patients with acute pain who reported a verbal numeric rating scale (VNRS) pain score greater than or equal to 5. Exclusion criteria were younger than 18 years, known ketamine intolerance, nontraumatic chest pain, altered mental status, pregnancy, and nasal occlusion. Patients received usual paramedic care and were randomized to receive either intranasal ketamine or intranasal saline solution at 0.75 mg/kg. The primary outcome was the proportion of patients with VNRS score reduction greater than or equal to 2 at 30 minutes. Secondary outcomes were pain reduction at 15 minutes, patient-reported comfort, satisfaction scores, nitrous oxide consumption, and incidence of adverse events. RESULTS: One hundred twenty subjects were enrolled. Seventy-six percent of intranasal ketamine patients versus 41% of placebo patients reported a greater than or equal to 2-point VNRS reduction at 30 minutes (difference 35%; 95% confidence interval 17% to 51%). Median VNRS reduction at 15 minutes was 2.0 and 1.0 and at 30 minutes was 3.0 and 1.0 for ketamine and placebo, respectively. Improved comfort at 15 and 30 minutes was reported for 75% versus 57% and 61% versus 46% of ketamine and placebo patients, respectively. Sixty-two percent of patients (95% confidence interval 49% to 73%) versus 20% (95% confidence interval 12% to 32%) reported adverse events with ketamine and placebo, respectively. Adverse events were minor, with no patients requiring physical or medical intervention. CONCLUSION: Added to nitrous oxide, intranasal ketamine provides clinically significant pain reduction and improved comfort compared with intranasal placebo, with more minor adverse events.
Web site Editorial comments:
This is a well designed RCT in the EMS setting comparing IN ketamine to
placebo in patients with pain scores over 5. IN ketamine was not just
used in trauma patients like most pain studies, instead they included a
variety of different causes of pain making it applicable to a broader
EMS population. Using a relatively low dose of IN ketamine (0.75 mg/kg)
they found clinically significant decreases in pain scores in 76% of
cases (compared to 41% in placebo arm).
________________________________________________________________
Blancher, M., M. Maignan,
et al. (2019). "Intranasal sufentanil versus intravenous morphine for
acute severe trauma pain: A double-blind randomized non-inferiority
study." PLoS Med 16(7):
e1002849.
Abstract:
BACKGROUND: Intravenous morphine (IVM) is the most common strong
analgesic used in trauma, but is associated with a clear time limitation
related to the need to obtain an access route. The intranasal (IN) route
provides easy administration with a fast peak action time due to high
vascularization and the absence of first-pass metabolism. We aimed to
determine whether IN sufentanil (INS) for patients presenting to an
emergency department with acute severe traumatic pain results in a
reduction in pain intensity non-inferior to IVM. METHODS AND FINDINGS:
In a prospective, randomized, multicenter non-inferiority trial
conducted in the emergency departments of 6 hospitals across France,
patients were randomized 1:1 to INS titration (0.3 mug/kg and additional
doses of 0.15 mug/kg at 10 minutes and 20 minutes if numerical pain
rating scale [NRS] > 3) and intravenous placebo, or to IVM (0.1 mg/kg
and additional doses of 0.05 mg/kg at 10 minutes and 20 minutes if NRS >
3) and IN placebo. Patients, clinical staff, and research staff were
blinded to the treatment allocation. The primary endpoint was the total
decrease on NRS at 30 minutes after first administration. The
prespecified non-inferiority margin was -1.3 on the NRS. The primary
outcome was analyzed per protocol. Adverse events were prospectively
recorded during 4 hours. Among the 194 patients enrolled in the
emergency department cohort between November 4, 2013, and April 10,
2016, 157 were randomized, and the protocol was correctly administered
in 136 (69 IVM group, 67 INS group, per protocol population, 76% men,
median age 40 [IQR 29 to 54] years). The mean difference between NRS at
first administration and NRS at 30 minutes was -4.1 (97.5% CI -4.6 to
-3.6) in the IVM group and -5.2 (97.5% CI -5.7 to -4.6) in the INS
group. Non-inferiority was demonstrated (p < 0.001 with 1-sided
mean-equivalence t test), as the lower 97.5% confidence interval of 0.29
(97.5% CI 0.29 to 1.93) was above the prespecified margin of -1.3. INS
was superior to IVM (intention to treat analysis: p = 0.034), but
without a clinically significant difference in mean NRS between groups.
Six severe adverse events were observed in the INS group and 2 in the
IVM group (number needed to harm: 17), including an apparent imbalance
for hypoxemia (3 in the INS group versus 1 in the IVM group) and for
bradypnea (2 in the INS group versus 0 in the IVM group). The main
limitation of the study was that the choice of concomitant analgesics,
when they were used, was left to the discretion of the physician in
charge, and co-analgesia was more often used in the IVM group. Moreover,
the size of the study did not allow us to conclude with certainty about
the safety of INS in emergency settings. CONCLUSIONS: We confirm the
non-inferiority of INS compared to IVM for pain reduction at 30 minutes
after administration in patients with severe traumatic pain presenting
to an emergency department. The IN route, with no need to obtain a
venous route, may allow early and effective analgesia in emergency
settings and in difficult situations. Confirmation of the safety profile
of INS will require further larger studies. TRIAL REGISTRATION:
ClinicalTrials.gov NCT02095366. EudraCT 2013-001665-16.
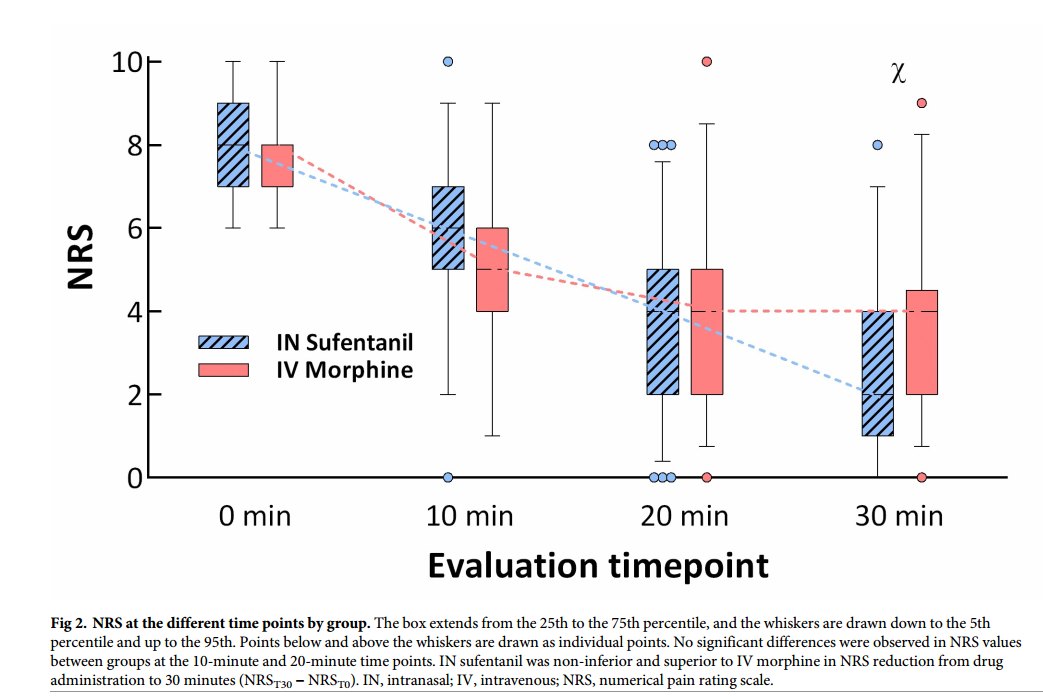
Graph from Blancher et al showing the reduction in pain scores (NRS –
numerical pain reduction score) for IV morphine and IN sufentanil in
patients suffering severe pain due to traumatic injuries.
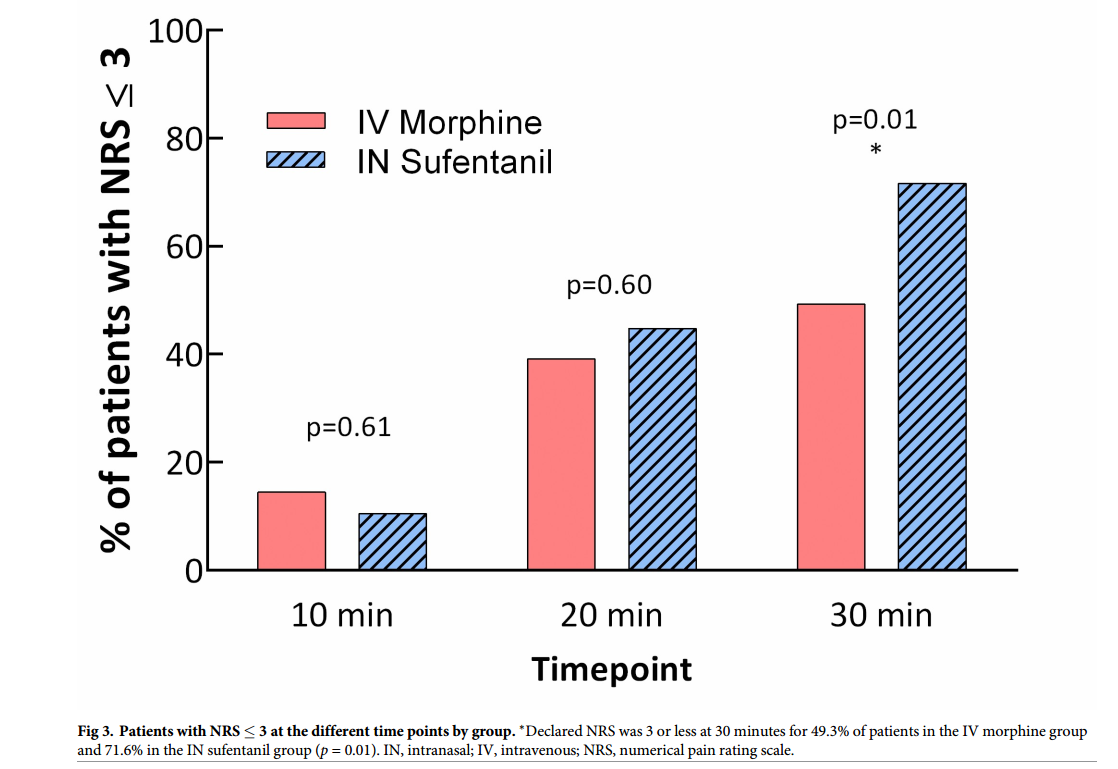
Graph from Blancher et al showing the percentage of patients over time
for whom their pain scores become less than 3 on the pain scale. This
study allowed titration of IV morphine or IN sufentanil in patients
suffering severe pain due to traumatic injuries. The key point being
that you can easily and safely titrate pain to whatever level you desire
using IN sufentanil.
Web site Editorial comments:
This is one of several recent prospective randomized trials comparing IV
morphine to intranasal sufentanil in adults with moderate to severe
pain. They included trauma patients with pain scores > 6 on a 10 point
scale. This study differs from the study by Sin, et al, in that it was
done in Europe, it was multicenter and it allowed titration of the
medications to effective pain control levels. They found sufentanil to
be slightly superior to IV morphine for pain control by 30 minutes
(equivalent at 10 and 20 minutes), (though because titration was allowed
it seems they simply needed to give more medication should they want
lower pain scores.) They
also found a few minor incidences of hypoxemia in both groups but more
in the sufentanil group (3 of 67 versus 1 of 69). They conclude that
titrating sufentanil via the IN route allows early, safe and effective
analgesia in emergency settings and difficult situations.
This study supports the growing body of evidence noting IN sufentanil is
equivalent to IV morphine. It also demonstrates what all of us who use
this formulation know – hypoxemia is a risk with sufentanil (unlike
fentanyl where it is not described in standard doses) and therefore
pulse oximetry and close observation are mandatory. Finally they use a
treatment that all of us should use for pain when using IV drugs or IN
drugs – titration to effect.
The drug works so rapidly that it is easy to titrate so start a
little low and titrate to effect to avoid respiratory depression. I have
been using this therapy for well over a decade and find it extremely
effective, very rapid in onset, a great patient satisfier, but
occasionally patients develop hypoxemia and vertigo – especially the
elderly.
Open Access free article: https://journals.plos.org/plosmedicine/article?id=10.1371/journal.pmed.1002849
Open Access free article: Click here for PDF
________________________________________________________________
Lemoel, F., J. Contenti,
et al. (2019). "Intranasal sufentanil given in the emergency department
triage zone for severe acute traumatic pain: a randomized double-blind
controlled trial." Intern Emerg Med
14(4): 571-579.
Abstract: The goal of our study was to determine if an intranasal (IN) dose of sufentanil delivered in the ED triage zone would improve the management of severely painful patients. We performed a randomized, double blind and placebo-controlled trial on adult patients suffering from an acute severe pain (>/= 6/10) consecutive to an isolated limb injury. We compared 2 analgesic strategies: the usual pain treatment with IV-only multimodal analgesics (IVMA) including IV opioids if needed (control group) and another strategy (active group) based on a single dose of IN sufentanil (0.4 mug/kg) given at triage and followed by IV multimodal analgesia. Our primary outcome was the proportion of patients reaching pain-relief (</= 3/10) 30 min after IN injection at triage. Secondary outcomes were rates of adverse events, frequency of clinical interventions required by these events, and satisfaction of patients. A total of 144 adult participants completed the study, 72 in each group. Compared with usual IV-only pain management, the analgesic strategy initiated in triage zone with a dose of IN sufentanil increased the proportion of patients reaching pain relief in 30 min: 72.2% versus 51.4%, in our trial (p = 0.01 and number needed to treat of 5). There was no serious adverse event (AE) in both groups. Patients who received IN sufentanil experienced more frequently minor opiate side effects. Proportion of respiratory AEs was higher in the active group (12.5% of bradypnea < 10 cycles per minute versus 1.4%) but these events were of mild severity, as only 2 participants (one in each group) received temporary low dose oxygen therapy, and none required naloxone. Lengths of stay in the ED were similar in both groups, as well as satisfaction of patients (above 9/10) and pain scores at discharge (< 2/10). We found that a single dose of IN sufentanil delivered in the ED triage zone significantly increases the proportion of severely painful patients reaching pain relief in 30 min, compared to usual analgesia with IV-only multimodal analgesia.
Web site Editorial comments:
These authors randomized 144 patients suffering severe traumatic limb pain
(>6/10) to two arms: One got IN sufentanil in triage plus IV multimodal
analgesia as needed, the second got only IV multimodal analgesia.
Pain control and patient satisfaction
were the same by ED discharge, however the IN sufentanil group
had far high pain control (pain less than3/10) at 30 minutes from
arrival than the IV group:
72% versus 52%. One patient in each group required supplemental oxygen.
We have seen IN
fentanyl used at triage for over a decade in pediatric hospitals, now we
have data showing its rapid efficacy in adult trauma patients. If you
want to get good control of pain in less than 30 minutes from arrival
this therapy is extremely effective, especially if you titrate to
effect. Be smart though – use pulse oximetry on these patients and be
careful in the elderly.
________________________________________________________________
Sin, B., I. Jeffrey, et
al. (2019). "Intranasal Sufentanil Versus Intravenous Morphine for Acute
Pain in the Emergency Department: A Randomized Pilot Trial." J Emerg
Med 56(3): 301-307.
Abstract:
BACKGROUND: Patients in the United States frequently seek medical attention
in the emergency department (ED) to address their pain. The intranasal (i.n.)
route provides a safe, effective, and painless alternative method of
drug administration. Sufentanil is an inexpensive synthetic opioid with
a high therapeutic index and rapid onset of action, making it an
attractive agent for management of acute pain in the ED. OBJECTIVE: The
objective of our study was to evaluate the safety and efficacy of i.n.
sufentanil as the primary analgesic for acute pain in the ED. METHODS:
This was a single-center, prospective, randomized, double-blind,
double-dummy, controlled trial that evaluated the use of i.n. sufentanil
0.7 mug/kg via mucosal atomizer device vs. intravenous morphine 0.1
mg/kg in adult patients who presented to the ED with acute pain. The
primary outcome was patient's pain score at 10 min after administration
of intervention. Secondary outcomes were adverse events, the need for
rescue analgesia, and patient satisfaction after treatment. RESULTS:
Thirty patients were enrolled in each group. There was no significant
difference in pain scores at 10 min after administration of intervention
(sufentanil: 2.0, interquartile range = 2.0-3.3 vs. morphine: 3.0,
interquartile range = 2.0-5.3, p = 0.198). No serious adverse events
were reported. Rescue analgesia was not requested in either group. No
significant difference in median satisfaction scores was found.
CONCLUSION: The use of i.n. sufentanil at 0.7 mug/kg/dose resulted in
rapid and safe analgesia with comparable efficacy to i.v. morphine for
up to 30 min in patients who presented with acute pain in the ED.
Web site Editorial comments:
This prospective randomized trial compared IV morphine to IN sufentanil
in adults with moderate to severe pain. They found that the two
therapies were equivalent (pain scores decreasing from 9/10 to 2/10 for
morphine and from 10/10 to 2/10
for sufentanil in 5 minutes.) There were infrequent adverse events with
none being serious.
What more can I say – IN sufentanil is just as good (or
better) than IV morphine in adults and its faster (see other studies
featured here on that topic). Ditto for IN fentanyl in children. Now IN
ketamine has come along and is nearly as good but does not require using
an opiate. There is so much data on this topic, yet even in 2020 most
clinicians don’t use these treatment modalities in their practice. If
you are reading this you should teach your colleagues – this will give
them another tool in the toolbox of pain control and it is usually
faster, less expensive and less resource intensive than standard IV
treatments.
________________________________________________________________
Frey, T. M., T. A. Florin,
et al. (2019). "Effect of Intranasal Ketamine vs Fentanyl on Pain
Reduction for Extremity Injuries in Children: The PRIME Randomized
Clinical Trial." JAMA Pediatr
173(2): 140-146.
Abstract:
Importance: Timely analgesia is critical for children with injuries
presenting to the emergency department, yet pain control efforts are
often inadequate. Intranasal administration of pain medications provides
rapid analgesia with minimal discomfort. Opioids are historically used
for significant pain from traumatic injuries but have concerning adverse
effects. Intranasal ketamine may provide an effective alternative.
Objective: To determine whether intranasal ketamine is noninferior to
intranasal fentanyl for pain reduction in children presenting with acute
extremity injuries. Design, Setting, and Participants: The Pain
Reduction With Intranasal Medications for Extremity Injuries (PRIME)
trial was a double-blind, randomized, active-control, noninferiority
trial in a pediatric, tertiary, level 1 trauma center. Participants were
children aged 8 to 17 years presenting to the emergency department with
moderate to severe pain due to traumatic limb injuries between March
2016 and February 2017. Analyses were intention to treat and began in
May 2017. Interventions: Intranasal ketamine (1.5 mg/kg) or intranasal
fentanyl (2 microg/kg). Main Outcomes and Measures: The primary outcome
was reduction in visual analog scale pain score 30 minutes after
intervention. The noninferiority margin for this outcome was 10.
Results: Of 90 children enrolled, 45 (50%) were allocated to ketamine
(mean [SD] age, 11.8 [2.6] years; 26 boys [59%]) and 45 (50%) to
fentanyl (mean [SD] age, 12.2 [2.3] years; 31 boys [74%]). Thirty
minutes after medication, the mean visual analog scale reduction was
30.6 mm (95% CI, 25.4-35.8) for ketamine and 31.9 mm (95% CI, 26.6-37.2)
for fentanyl. Ketamine was noninferior to fentanyl for pain reduction
based on a 1-sided test of group difference less than the noninferiority
margin, as the CIs crossed 0 but did not cross the prespecified
noninferiority margin (difference in mean pain reduction between groups,
1.3; 90% CI, -6.2 to 8.7). The risk of adverse events was higher in the
ketamine group (relative risk, 2.5; 95% CI, 1.5-4.0), but all events
were minor and transient. Rescue analgesia was similar between groups
(relative risk, 0.89; 95% CI, 0.5-1.6). Conclusions and Relevance:
Ketamine provides effective analgesia that is noninferior to fentanyl,
although participants who received ketamine had an increase in adverse
events that were minor and transient. Intranasal ketamine may be an
appropriate alternative to intranasal fentanyl for pain associated with
acute extremity injuries. Ketamine should be considered for pediatric
pain management in the emergency setting, especially when opioids are
associated with increased risk. Trial Registration: ClinicalTrials.gov
Identifier: NCT02778880.
Web site Editorial comments:
This study is a prospective randomized trial that compared the efficacy
of IN ketamine (1.5 mg/kg) to IN fentanyl (2 ug/kg) in children with
extremity injuries. They gave adequate doses of the medications bases on
prior research and found that the two treatment modalities resulted in
equivalent reductions in pain scores (about 3 out of 10). There were
slightly more minor adverse events (dizzy, drowsy, bad taste) in the
ketamine group but no serious adverse events.
We already know from RCT’s 10 years ago that IN fentanyl is
equivalent to IV morphine for treating moderate to severe pain in the
emergency room. We also know the IN route results in faster pain relief
and more treatment of patients that require strong pain medications.
This study (among many with similar results) now shows us that in a
situation where you might want to avoid an opiate, there is an
equivalent alternative – nasal ketamine.
________________________________________________________________
Mozafari, J., M. Maleki Verki, et al. (2019). "Comparing intranasal
ketamine with intravenous fentanyl in reducing pain in patients with
renal colic: A double-blind randomized clinical trial." Am J Emerg
Med.
Nazemian, N., M. Torabi, et al. (2019).
"Atomized intranasal vs intravenous fentanyl in severe renal colic pain
management: A randomized single-blinded clinical trial." Am J Emerg
Med: 158483.
Abstract Mozafari:
BACKGROUND: Kidney stones are a fairly common problem that manifests itself
as symptoms of acute abdominal and flank pains in patients presenting to
emergency departments. OBJECTIVE: The present study was conducted to
compare the analgesic effect of intravenous fentanyl with that of
intranasal ketamine in renal colic patients. METHODS: One mg/kg of
intranasal ketamine was administered in the first group, and one mug/kg
of intravenous fentanyl in the second group. The pain severity was
measured in the patients in terms of a visual analogue scale (VAS) score
at the beginning of the study and at minutes 5, 15 and 30, and the
medication side-effects were evaluated and recorded. RESULTS: A total of
130 patients were ultimately assessed in two groups of 65. In the
ketamine group, the mean severity of pain was 8.72+/-1.52 at the
beginning of the study (P<0.001), 5.5+/-2.97 at minute 5 (P<0.001),
3.38+/-3.35 at minute 15 (P=0.004) and 2.53+/-3.41 at minute 30
(P=0.449). In the fentanyl group, this severity was 9.66+/-88.8 in the
beginning of the study (P<0.001), 7.27+/-1.37 at minute 5 (P<0.001),
4.61+/-1.5 at minute 15 (P=0.004) and 1.24+/-1.25 at minute 30
(P=0.449). The general prevalence of the medication side-effects was 10
(15.4%) in the ketamine group and 1 (1.5%) in the fentanyl group
(P=0.009). CONCLUSIONS: Ketamine was found to be less effective than
fentanyl in controlling renal colic-induced pain, and to be associated
with a higher prevalence of side-effects; nevertheless, ketamine can be
effective in controlling this pain in conjunction with other
medications.
These authors found that a somewhat low dose of nasal ketamine of 1
mg/kg (given the pain severity) effectively reduced pain associated with
renal colic from a mean score of 8.7 down to 2.5 in 30 minutes. This was
slightly less effective than IV fentanyl (9.7 to 1.3) but did not
require an IV.
Abstract Nazemian:
OBJECTIVES: Renal colic is one of the most common painful disorders in
patients referred to the emergency department. The main purpose of this
study was to compare the efficiency of two methods of intravenous (IVF)
and intranasal (INF) fentanyl administration in pain management in
patients with severe renal colic. MATERIALS & METHODS: This was a
single-blind randomized clinical trial performed on patients with severe
renal colic. The severity of pain was >/=8 based on the Numerical Rating
Scale (NRS). The efficacy of pain management was compared within and
between the IVF (intramuscular Ketorolac + intravenous fentanyl) and INF
(intramuscular Ketorolac + intranasal fentanyl) groups at different
times points. Oral consent was obtained from all the patients. RESULTS:
Of 220 individuals, 96 (43.60%) were women and 124 (56.40%) were men.
There were no significant differences between the two groups regarding
the baseline pain severity, age, sex, history of urolithiasis and body
mass index (BMI). The pain severity showed a significant reducing trend
in both groups (p<0.0001). There was also a significant difference
comparing the mean pain severity between groups at different times
(p<0.0001). In each group, the severity of pain showed significant
reduction compared with its prior measurement (P<0.0001). CONCLUSION:
Fentanyl is highly effective in controlling pain in patients with severe
renal colic referring to the emergency department. Intranasal
administration of fentanyl combination with ketorolac can be an
appropriate, non-invasive, easy-to-use and fast alternative to the
intravenous method to manage pain in these patients.
These authors showed that IN fentanyl was as effective as IV fentanyl
for reducing renal colic pain when both were combined with ketorolac.
Web site Editorial comments:
Both of these
studies (and prior studies by Belkouch 2015 and Farnia 2017)
point to a less resource intense
way to treat renal colic: Transmucosal medication delivery o intranasal
opiates (fentanyl or sufentanil) or intranasal ketamine. With the
availability these analgesic options and of transmucosal antiemetics and
intranasal or oral ketorolac/ibuprofen it is possible to treat renal
colic with no IV access and with far less resource utilization.
With the current US focus on maximizing billing it is unlikely
this idea will be adopted by many emergency rooms in the US (which might
explain why these studies come from outside the US), never the less it
is a great idea for less resource intense setting all over the world or
in settings where you do not wish to waste resources.
Pubmed link Nazemian: https://www.ncbi.nlm.nih.gov/pubmed/31740092
________________________________________________________________
Bregstein, J. S., A. M. Wagh, et al. (2019).
"Intranasal Lorazepam for Treatment of Severe Agitation in a Pediatric
Behavioral Health Patient in the Emergency Department."
Ann Emerg Med.
Abstract: The treatment of severe agitation, aggression, and violent behavior in behavioral health patients who present to the emergency department (ED) often requires the intramuscular administration of a sedative. However, administering an intramuscular sedative to an uncooperative patient is associated with the risk of needlestick injuries to both patients and health care providers, and times to onset of sedation range from 15 to 45 minutes. Intranasal absorption is more rapid than intramuscular, with sedatives such as lorazepam reaching peak serum concentrations up to 6 times faster when administered intranasally. We present the first report of using intranasal lorazepam as a needle-free method of providing rapid and effective sedation to treat severe agitation in a pediatric behavioral health patient presenting to the ED.
Web site Editorial comments:
This is a case report of a child with a violent
behavioral disorder who required chemical restraint but refused oral or
buccal medications. His mother asked for something other than an IM
injection due to his prior experiences with them and the fear they
induced. Furthermore the staff were concerned about needle stick risks
given his aggressive behavior. Therefore he was held down and
administered 0.05 mg/kg of intranasal lorazepam. He was calm in 5
minutes and this lasted about 3 hours. He required an additional dose
which was increased to 0.1 mg/kg and this put him to sleep and kept him
calm for hours.
Commentary: I put this case
report in featured articles to stimulate interest in a “new” idea for
intranasal medications – sedation of the agitated and or delirious
patient. I started to use IN lorazepam for sedation in adults about 15
years ago. Fortunately or unfortunately at that point in my career I
rarely needed to sedate agitated patients so I did not gain enough
experience to really pontificate on the topic.
I can say that I start with 2 mg dose (not weight based) – half
up each nostril. It is viscous so does not atomize well, but the nasal
atomizer made it much simpler to control and deliver in these difficult
patients (compared to dripping it) so that was my method of delivery.
It worked well – sometimes I thought too well as the patients
went quickly to sleep for hours – perhaps because they already had many
other drugs and/or alcohol on board. It is an area wide open for
research and if we knew the right dose that worked and was safe (from a
dosing escalation study) it would be invaluable in the EMS setting as
well as in many other situations where difficult patients are cared for.
Maybe one of you readers is interested in doing this simple study – you
could milk it for multiple publications – dosing study, validation
study, EMS study.
________________________________________________________________
Khalil, W. and N. Raslan (2019). "The effectiveness of topical lidocaine
in relieving pain related to intranasal midazolam sedation: a
randomized, placebo-controlled clinical trial." Quintessence Int:
2-7.
Abstract:
OBJECTIVE: Intranasal midazolam (INM) is an increasingly popular agent for
sedation in the emergency department and outside the hospital in
physician, imaging, and dental offices, to facilitate diagnostic and
minor surgical procedures and avoid the need for an operating room and
general anesthesia. The use of INM has been commonly associated with a
burning sensation of the nasal mucosa. Despite its significance, this
subject has received little adequate research focus. The objective of
the current study was to evaluate the effectiveness of topical lidocaine
in relieving pain related to INM administration. METHOD AND MATERIALS:
This was a blinded, randomized placebo-controlled trial. Sixty-three
uncooperative children undergoing dental treatment, aged 4 to 11 years,
were randomly assigned to one of three groups to receive the drug
nasally via a precalibrated spray as per the following assignments:
group A received 0.5 mg/kg midazolam, group B received lidocaine 2%
prior to 0.5 mg/kg midazolam, and group C received saline 0.9%
(placebo), 0.5 mg/kg. Children were asked to record the degree of pain
using the Wong-Baker faces scale. Parental acceptance was also rated.
RESULTS: Topical lidocaine prior to INM administration reduced the
burning sensation in the nasal mucosa and improved the drug acceptance.
The median score of pain was 8, 1, and 4 in groups A, B, and C,
respectively. The differences among the three groups were statistically
significant (P > .05). The children's acceptance and parents' future
acceptance regarding the intranasal drug administration was
significantly higher in group B. CONCLUSION: INM administration results
in burning sensation in the nasal mucosa with high levels of pain. Using
topical lidocaine 2% counteracted the burning sensation and achieved a
higher acceptance rate and lower pain scores.
Web site Editorial comments:
This study reconfirmed that pretreatment with lidocaine dramatically
reduces pain and increases parental acceptance of intranasal midazolam.
Pain scores without lidocaine were 8/10 whereas they were 4/10 with
lidocaine.
Comment: Despite
15 years of literature on this issue – all referenced in these web pages
in the sedation section - many clinician still don’t pre-treat with
lidocaine. I don’t get it. Its simple, effective, improves satisfaction
and reduces stress for everyone involved. I will continue to point out
these articles until it becomes standard care to include lidocaine on
every nasal midazolam sedation case. Just do it.
________________________________________________________________
Malia, L., V. M. Laurich, et al. (2019). "Adverse events and
satisfaction with use of intranasal midazolam for emergency department
procedures in children." Am J Emerg Med
37(1): 85-88.
Abstract: PURPOSE: Procedural sedation is commonly performed in the emergency department (ED). Having safe and fast means of providing sedation and anxiolysis to children is important for the child's tolerance of the procedure, parent satisfaction and efficient patient flow in the ED. OBJECTIVE: To evaluate fasting times associated with the administration of intranasal midazolam (INM) and associated complications. Secondary objectives included assessing provider and caregiver satisfaction scores. METHODS: A prospective observational study was conducted in children presenting to an urban pediatric emergency department who received INM for anxiolysis for a procedure or imaging. Data collected included last solid and liquid intake, procedure performed, sedation depth, adverse events and parent and provider satisfaction. RESULTS: 112 patients were enrolled. The mean age was 3.8years. There were no adverse events experienced by any patients. Laceration repair was the most common reason for INM use. The median depth of sedation was 2.0 (cooperative/tranquil). The median liquid NPO time was 172.5min and the median NPO time for solids was 194.0min. 29.8% were NPO for liquids </=2h and 62.5% were NPO for solids </=2h. Parent and provider satisfaction was high: 90.4% of parents' and 88.4% of providers' satisfaction scores were a 4 or 5 on a 5 point Likert scale. CONCLUSION: Our data suggest that short NPO of both solids and liquids are safe for the use of INM. Additionally, parent and provider satisfaction scores were high with the use of INM.
Web site Editorial comments:
Concern occasionally arises regarding the NPO status of a child and the
safety of IN medications. These authors looked into this in ED patients
undergoing sedation with IN midazolam.
They found that 2/3 of patients had eaten within the last 2 hours
yet no patient in their stud (112 patients) suffered from aspiration.
Comments: Emergency room procedures
frequently occur in suboptimal conditions and NPO status is usually
unknown or the patient has recently eaten or consumed liquids. Never the
less, the IN sedation literature demonstrates some nausea and vomiting
associated with dexmedetomidine (<1% generally) but
has no reports of aspiration to
my knowledge. This is likely due to the fact that IN medications do not
cause general anesthesia or even deep sedation – they generally put the
patient into a calm or sleepy state but they are easily aroused (as was
the case in this paper). In
this condition the patient can protect their airway so NPO status is not
really relevant. If you
really want “un" -conscious sedation then you should put in an IV, have
suction equipment available, get them on monitors and put them down.
Intranasal medications are the wrong tools for this type of sedation.
________________________________________________________________
Ryan, P. M., A. J.
Kienstra, et al. (2019). "Safety and effectiveness of intranasal
midazolam and fentanyl used in combination in the pediatric emergency
department." Am J Emerg Med
37(2): 237-240.
Abstract: OBJECTIVE: To examine the safety and effectiveness of intranasal midazolam and fentanyl used in combination for laceration repair in the pediatric emergency department. METHODS: We performed a retrospective chart review of a random sample of 546 children less than 18years of age who received both intranasal midazolam and fentanyl for laceration repair in the pediatric emergency department at a large, urban children's hospital. Records were reviewed from April 1, 2012 to June 31, 2015. The primary outcome measures were adverse events and failed laceration repair. RESULTS: Of the 546 subjects analyzed, 5.1% had multiple lacerations. Facial lacerations were the most common site representing 70.3%, followed by lacerations to the hand (9.9%) and leg (7.0%). The median length of lacerations was 1.5cm [1.0-2.5]. The median dose of fentanyl was 2.0mug/kg [1.9-2.0] and midazolam was 0.2mg/kg [0.19-0.20]. There were no serious adverse events reported. The rate of minor side effects was 0.7% (95% CI 0.2% to 1.9%); 0.5% (95% CI 0.1% to 1.6%) experienced anxiety and 0.2% (95% CI 0.0% to 1.0%) vomited. No patients developed hypotension or hypoxia. Of the 546 patients, 2.4% (95% CI 1.3% to 4.0%) experienced a treatment failure. 2.0% (95% CI 1.3% to 4.0%) required IV sedation and 0.4% (95% CI 0.0% to 1.3%) were repaired in the operating room. CONCLUSIONS: Our results suggest that the combination of INM and INF may be a safe and effective strategy for procedural sedation in young children undergoing simple laceration repair.
Web site Editorial comments:
This study looked at the effectiveness of combined nasal midazolam and
fentanyl for procedural sedation during laceration repair. They found it
was 96.7% effective in allowing them to complete their procedure (much
higher than prior studies that only used midazolam for sedation). They
believe this is due to using the combined treatment. They had no serious
adverse events (respiratory depression or hypotension).
Although this study is retrospective in nature and it used a
fairly low dose of midazolam (0.2 mg/kg) for sedation I chose it for a
few reasons. First it points out that combined therapy is often better
than single drug therapy – something we often do when using IV drugs but
for some reason forget using nasal drugs. Second – it reduces the down
side of midazolam for sedation. Midazolam alone is really only an
anxiolytic, not a great sedative, it has no analgesic properties and it
burns when applied. Adding fentanyl helps by providing pain control to
suppress the burning and suppress any pain that occurs with wound
injection and suturing.(They should pretreat with lidocaine however)
Lastly they had super good results – only 2.4% failure in sedation –
showing how effectively you can sedate a patient without using an IV.
I use this combo (or midazolam plus sufentanil) all the time for
procedural sedation in kids and adults and I titrate to effect so I
really do not have failures as I give enough drug slowly to sedate
whomever I am working on – just be patient and give enough – the dose is
irrelevant as long as it is enough but not too much. Combo therapy with
IN drugs is very effective and markedly reduces need for nursing
interventions and long term monitoring. This web site features studies
from Africa where they do this to conduct major procedures and surgeries
so it can be very effective if you need it to be.
________________________________________________________________
Gu, H. B., Y. A. Song, et al. (2019). "Median Effective Dose of
Intranasal Dexmedetomidine for Transthoracic Echocardiography in
Children with Kawasaki Disease Who Have a History of Repeated Sedation."
Med Sci Monit 25: 381-388.
Abstract: BACKGROUND The aim of this study was to investigate the median effective dose (ED50) of intranasal dexmedetomidine for echocardiography in children with Kawasaki disease who had a history of repeated sedation. MATERIAL AND METHODS There were 73 pediatric Kawasaki disease patients aged 1 to 36 months enrolled in this study who had American Society of Anesthesiologists (ASA) I-II, were scheduled to undergo echocardiography under sedation. They were assigned to 2 groups (group A: age 1-18 months, and group B: age 19-36 months). Intranasal dexmedetomidine was administered before echocardiography. The dose of intranasal dexmedetomidine was determined with the up-down sequential allocation, and the initial dose was 2 mug/kg with an increment/decrement of 0.2 mug/kg. The ED50 of intranasal dexmedetomidine for sedation was determined with the up-and-down method of Dixon and Massey and probit regression. The time to effective sedation, time to regaining consciousness, vital signs, oxygen saturation, echocardiographic examination time, clinical side-effects, and characteristics of regaining consciousness were recorded and compared. RESULTS The ED50 of intranasal dexmedetomidine for sedation was 2.184 mug/kg (95% CI, 1.587-2.785) in group A and 2.313 mug/kg (95% CI, 1.799-3.426) in group B. There were no significant differences in the time to sedation and time to regaining consciousness between groups. Additionally, change in hemodynamic and hypoxemia were not noted in both groups. CONCLUSIONS The ED50 of intranasal dexmedetomidine was determined in children with Kawasaki disease who had a history of repeated sedation to be appropriate for repeated-routine sedation of echocardiographic examination in pediatric patients. The ED50 of intranasal dexmedetomidine for echocardiography in this circumstance is similar to that in children receiving initial sedation.
This study found that the median effective dose of intranasal
dexmedetomidine for children less than 3 years old who required sedation
for a transthoracic echo was about 2.2 ug/kg.
Pubmed link: https://www.ncbi.nlm.nih.gov/pubmed/30636258
Yang, F., S. Li, et
al. (2019). "Fifty Percent Effective Dose of Intranasal Dexmedetomidine
Sedation for Transthoracic Echocardiography in Children With Cyanotic
and Acyanotic Congenital Heart Disease." J Cardiothorac Vasc Anesth.
Abstract: OBJECTIVES: To determine the 50% and 95% effective dose of intranasal dexmedetomidine sedation for transthoracic echocardiography in children with cyanotic and acyanotic congenital heart disease. DESIGN: A prospective, nonrandomized study. SETTING: A tertiary care teaching hospital. PARTICIPANTS: Patients younger than 18 months with known or suspected congenital heart disease scheduled for transthoracic echocardiography with sedation. INTERVENTIONS: Patients were divided into a cyanotic group (blood oxygen saturation <85%) or an acyanotic group (blood oxygen saturation >/=85%). This study used Dixon's up-and-down method sequential allocation design. In both groups, the initial dose of intranasal dexmedetomidine was 2 mug/kg and the gradient of increase or decrease was 0.25 mug/kg. MEASUREMENTS AND MAIN RESULTS: The 50% effective dose (95% confidence interval) of intranasal dexmedetomidine sedation for transthoracic echocardiography was 3.2 (2.78-3.55) mug/kg and 1.9 (1.69-2.06) mug/kg in the cyanotic and acyanotic groups, respectively. None of the patients experienced significant adverse events. CONCLUSION: The 50% (95% confidence intervals) effective doses of intranasal dexmedetomidine sedation for transthoracic echocardiography were 3.2 (2.78-3.55) mug/kg and 1.9 (1.69-2.06) mug/kg in children with cyanotic and acyanotic congenital heart disease, respectively.
These authors determined the dose of intranasal dexmedetomidine that
was effective at least 50% of the time at sedating children for
echocardiography. They found it to be 2 ug/kg for children with
acyanotic heart disease and 3.2 ug/kg for those with cyanotic heart
disease.
Liu, H., M. Sun, et al.
(2019). "Determination of the 90% effective dose of intranasal
dexmedetomidine for sedation during electroencephalography in children."
Acta Anaesthesiol Scand 63(7):
847-852.
Abstract: BACKGROUND: The intranasal route of dexmedetomidine (DEX) administration is becoming increasingly popular for providing adequate sedation during short examinations in infants and children. However, data on the 90% effective dose (ED90) of intranasal DEX are rare in children under 3 years old. METHODS: This is a double-blind trial using a biased coin design up-and-down sequential method (BCD-UDM). Fifty-three children aged under 3 years old requiring DEX for EEG were included in our study. The first patient received 2.5 mug kg(-1) DEX, and the dose of DEX administered to the subsequent patient was determined by the response of the previous patient. The patient responses were recorded and analysed to calculate the ED90 by isotonic regression. The 95% confidence interval (CI) was estimated using a bootstrapping method. RESULTS: Fifty-three patients were included in our study, of which 45 patients were successfully sedated, and the 8 instances of failed sedation were rescued using sevoflurane inhalation, allowing the completion of the procedure. The 90% effective dose of DEX was calculated to be 3.28 microg kg(-1) , and the 95% CI was 2.74 ~ 3.39 microg kg(-1) . No significant adverse events occurred in any of the patients. CONCLUSION: The 90% effective dose of intranasal DEX sedation for EEG was 3.28 mug kg(-1) in children under 3 years old.
This study found that the dose of intranasal dexmedetomidine that was
effective 90% in at least 90% of children less than 3 years old who
required sedation for an EEG was 3.28 ug/kg.
Comments: I have included three
studies here that describe the 50% and 90% effective dose of intranasal
dexmedetomidine for sedating children. Two studies are complicated by
the fact that the kids have heart disease. Never the less the data is
fairly consistent and can help us determine an adequate dose to reliably
sedate children. That dose is 2-3 mcg/kg.
Many studies use 1 to 1.5 ug/kg which is probably too low if you
want to reliably use this drug for sedation. 2-3 ug/kg is better and 3
ug/kg is likely best if you want a bit deeper and longer sedation.
A very common mistake when learning to use intranasal medications
is to under dose for fear of side effects. We now have hundreds of
studies on IN dexmedetomidine and it is very safe at adequate doses – so
don’t be fearful and give the right dose based on your needs of depth,
length of procedure and patient stimulation that will occur (the dose
will be higher than the IV dose) so you will be successful.
________________________________________________________________
Lei, H., L. Chao, et al.
(2019). "Incidence and risk factors of bradycardia in pediatric patients
undergoing intranasal dexmedetomidine sedation." Acta Anaesthesiol
Scand.
Abstract:
BACKGROUND: Dexmedetomidine is widely used for non-invasive pediatric
procedural sedation. However, the hemodynamic effects of intravenous
dexmedetomidine are a concern. There has been a growing interest in the
application of intranasal dexmedetomidine as a sedative in children.
OBJECTIVE: To investigate the incidence of bradycardia in children
undergoing intranasal dexmedetomidine sedation and to identify the
associated risk factors. METHODS: Data pertaining to pediatric patients
who underwent intranasal dexmedetomidine sedation for non-invasive
investigations at the Kunming Children's Hospital between October 2017
and August 2018 were retrospectively analyzed. RESULTS: Out of 9984
children who qualified for inclusion, 228 children (2.3%) developed
bradycardia. The incidence of bradycardia in the group that received
additional dose of dexmedetomidine was higher than that in the group
that did not receive additional dose (9.2% vs 16.7%; P = .003). The
incidence of bradycardia in males was higher than that in females (2.6%
vs 1.8%; P = .007). On multivariate logistic regression, only male
gender showed an independent association with the occurrence of
bradycardia (odds ratio 1.48; 95% confidence interval 1.11-1.97; P =
.008). CONCLUSIONS: The overall incidence of bradycardia in children
after sole use of intranasal dexmedetomidine sedation was 2.3%. Male
children showed a 1.48-fold higher risk of bradycardia. However, the
blood pressure of the children who developed bradycardia was within the
normal range. Simple wake-up can effectively manage bradycardia induced
by intranasal dexmedetomidine sedation.
Web site Editorial comments:
This article investigated the incidence of bradycardia in 9984 pediatric
patients receiving intranasal dexmedetomidine. 2.3% developed
bradycardia but it was never of clinical importance and did not require
intervention.
Comment: I featured this article due
to the slight concern surrounding dexmedetomidine induced bradycardia.
This article specifically investigated this issue in a very large
patient cohort (9984 uses). While they confirmed bradycardia in 2.3%, it
was never clinically important and easily resolved with patient
stimulation. In a larger study by Yang (also featured in this 2019
section) 17948 patients were
sedated with intranasal dexmedetomidine. They only reported clinically
significant cardiac effects – 1 child with PAT developed PAT during the
sedation, 4 children (0.02%) developed heart rate/BP changes more than
20% outside the normal range. Both authors conclude that adverse events
and bradycardia are very rare.
________________________________________________________________
Yang, F., Y. Liu, et al. (2019). "Analysis of 17 948 pediatric patients
undergoing procedural sedation with a combination of intranasal
dexmedetomidine and ketamine." Paediatr Anaesth
29(1): 85-91.
Abstract: BACKGROUND: Intranasal procedural sedation using dexmedetomidine is well described in the literature. The combination of intranasal dexmedetomidine and ketamine is a novel approach for which there are little data on the rate of successful sedation or adverse events. OBJECTIVES: The aim of this study is to evaluate the rate of successful sedation and adverse events of intranasal procedural sedation using a combination of dexmedetomidine and ketamine for diagnostic examination in children. METHODS: This was a retrospective study and data were collected after ethics approval. A total of 17 948 pediatric patients (7718 females, 10 230 males) in a tertiary hospital in China were evaluated. Patients received a combination of 2 mug kg(-1) of dexmedetomidine and 1 mg kg(-1) of ketamine intranasally for procedural sedation. The level of sedation and recovery was assessed by the Modified Observer Assessment of Alertness/Sedation scale and the Modified Aldrete Score. RESULTS: The rate of intranasal sedation success was 93% (16691/17948), intranasal sedation rescue was 1.8% (322/17948), and intranasal sedation failure was 5.2% (935/17948). Sedation success was defined as successful completed the diagnostic examination and obtained adequate diagnostic-quality images and reports. Intranasal sedation success, rescue and failure were respectively defined as sedation success with intranasal a single dose, additional bolus dose and the need for intravenous (IV) medications/inhalation agents. Median sedation time was 62 min (interquartile range: 55-70 min), median time for onset of sedation was 15 min (interquartile range: 15-20 min), and median sedation recovery time was 45 min (interquartile range: 38-53 min). Incidence of adverse events was low (0.58%; 105/17948), with major and minor adverse event being reported in 0.02% (4/17948) and 0.56% (101/17948) patients, respectively. Postoperative nausea and vomiting was the most common (0.3%; 53/17948) minor adverse event. CONCLUSION: Procedural sedation using a combination of intranasal dexmedetomidine and ketamine is associated with acceptable effectiveness and low rates of adverse events.
Web site Editorial comments:
This is a giant study reviewing the efficacy of IN dexmedetomidine (2 ug/kg)
plus IN ketamine (1 mg/kg) for procedural sedation in children. They
found the combination to provide effective sedation in 93% of cases with
onset of action in 15 minutes and length of sedation about 1 hour.
I chose this article due to its huge size and the information
we can glean from a single paper about efficacy and risks.
These authors used a combination
of medications and their procedures were primarily radiology based (not
painful, not stimulating to the patient) so their dose of
dexmedetomidine is probably right on at 2 ug/kg.
Prior studies have shown this to be the 50% effective dose for
stimulating procedures. Adding Ketamine probably helped a little to push
their efficacy up to 93%. Out of over 17 thousand cases they had 3 cases
that required airway interventions – all were known in advance to have
upper airway obstructive anatomy and so once sedated developed worsening
obstruction requiring intervention.
There were 4 minor events (out of nearly 18,000 uses!) related to
pulse or blood pressure for which they intervened pharmacologically so
bradycardia concerns are very minimal. Nausea and vomiting was the most
common adverse event occurring in 0.3% of patients, not resulting in
aspiration and easily controlled with ondansetron.
Free Open Access article: https://onlinelibrary.wiley.com/doi/full/10.1111/pan.13526
Free PDF: Yang, Dexmed in 18000 kids, Paed Anesth 2019
________________________________________________________________
Owusu, K. A., M. B. Dhakar,
et al. (2019). "Comparison of intranasal midazolam versus intravenous
lorazepam for seizure termination and prevention of seizure clusters in
the adult epilepsy monitoring unit." Epilepsy Behav
98(Pt A): 161-167.
Abstract: OBJECTIVE: The objective of the study was to compare the performance of intravenous (IV) lorazepam (IVL) and intranasal midazolam (INM) for seizure termination and prevention of seizure clusters in adults admitted to the epilepsy monitoring unit (EMU) in whom seizures were captured on continuous video-electroencephalogram. METHODS: Retrospective cohort of consecutive adults (>/=18years) with epilepsy admitted to the EMU at a single tertiary academic center, who experienced epileptic seizures (confirmed electroencephalographically) and required rescue therapy. The study spanned from January 2015 until December 2016, which included one year before and one year after transitioning from IVL to INM as the standard rescue therapy at our institution. RESULTS: A total of 50 subjects received rescue therapy and were included in the analysis. In the first year, out of 216 patients with epilepsy admitted to the EMU, 27 (13%) received IVL; in the second year, 23/217 (11%) received INM. There were no differences in baseline characteristics and markers of epilepsy severity, the median duration of index seizure (1.7min [interquartile range (IQR): 1.1-2.7] in IVL vs. 2.0min [IQR: 1.5-2.6] in INM group, p=0.20), or in the number of subjects requiring repeat benzodiazepine administrations (IVL 8/27 [29.6%] vs. INM 7/23 [30.4%], p=0.95). There were no differences in the median number of recurrent seizures in 24h (1 [IQR: 1-3] in IVL vs. 2 [IQR: 1-4] in INM, p=0.27), occurrence of status epilepticus (IVL 4/27 [14.8%] subjects vs. INM 1/23 [4.3%] subjects, p=0.36), incidence of seizure clusters (IVL 8/27 [29.6%] subjects vs. INM 7/23 [30.4%] subjects, p=0.95), need for transfer to an intensive care unit (ICU), or other adverse events. SIGNIFICANCE: In our retrospective study, INM was comparable with IVL for seizure termination and prevention of seizure clusters in the adult EMU. Intranasal midazolam circumvents the need for IV access to be maintained throughout hospitalization and is an attractive alternative to IVL as a rescue therapy in this setting. Ideally, future large, prospective, randomized, and double blind studies are needed to confirm these findings.
Web site Editorial comments:
This study compared the efficacy of IV lorazepam (1-2 mg) to Intranasal
midazolam (Generic - 3 mg or 0.6 ml atomized) in adult epilepsy patients
suffering acute (> 5 min) or cluster seizures (> 3 in 30 min) being
monitored in an epilepsy unit. They found that IN midazolam was
equivalent in efficacy but much easier to administer and the need for
maintaining an IV on the unit was eliminated. They have eliminated IV
lorazepam from their rescue medication and converted to IN Midazolam.
Comments: The
pediatric literature is replete with data showing the equivalence of IV
lorazepam and IN midazolam. Here is an adult study showing the same
results with a fairly low dose of nasal midazolam (3 mg midazolam in an
adult) against a standard 1-2 mg IV dose of lorazepam.
Several other studies have also been published this year noting
IN midazolam is effective in treating seizures in adults.
________________________________________________________________
Title:
Abstract:
Web site Editorial comments:
Pubmed link:________________________________________________________________
Title:
Abstract:
Web site Editorial comments:
Pubmed link:
________________________________________________________________
Title:
Abstract:
Web site Editorial comments:
Pubmed link:
________________________________________________________________
 Therapeutic
Intranasal Drug Delivery
Therapeutic
Intranasal Drug Delivery