Featured new articles related to intranasal drug delivery
January-December 2017:
Adelgais, K. M., A. Brent, et al. (2017).
"Intranasal Fentanyl and Quality of Pediatric Acute Care." J Emerg
Med.
Abstract:
BACKGROUND: Changes in the manner in which medications can be delivered
can have significant effects on the quality of care in the acute care
setting. OBJECTIVE: The objective of this study was to evaluate the
change in three Institute of Medicine quality indicators (timeliness,
safety, and effectiveness) in the pediatric emergency department (ED)
after the introduction of the Mucosal Atomizer Device Nasal (MADn) for
opioid analgesia. METHODS: This was a retrospective review of patients
receiving opioid analgesia for certain conditions over a 5-year period.
We compared patients receiving intravenous opioid (IVO) to those
receiving intranasal fentanyl (INF). Timeliness outcomes include time
from medication order to administration, time from dose to discharge,
overall time to analgesia, and ED length of stay. Effectiveness outcomes
include change in pain score and frequency of repeat dosing. Safety
outcomes were the frequency of reversal agent administration or a
documented oxygen desaturation of < 90%. Sensitivity analyses were
performed to evaluate the effect of moderate sedation on all three
outcomes. RESULTS: During the study period, 1702 patients received
opioid analgesia, 744 before and 958 after MADn introduction, of whom,
233 (24%) received INF. After MADn introduction, patients receiving INF
had a shorter time to discharge from dose (109 vs. 203 min; p < 0.05)
and shorter ED length of stay (168 vs. 267 min; p < 0.05). There was no
difference in pain score reduction; however, repeat dosing was less
frequent for patients receiving INF (16% vs. 27%). There was no use of
reversal medication and no difference in the frequency of oxygen
desaturation. When patients undergoing moderate sedation were removed
from the analysis, there was no difference in the direction of findings
for all three outcomes. CONCLUSIONS: INF is associated with improved
timeliness and equivalent effectiveness and safety when compared to IVO
in the setting of the pediatric ED.
Web site Editorial comments:
Here is another article showing that IN fentanyl is just as good as
IV opiates at controlling severe pain in a pediatric emergency
department but does not require nursing staff to establish any IV
access. This study is unique in that IN fentanyl
was used not just for orthopedic cases but was used for
any patient that needed opiates. Furthermore it results in faster
delivery of pain medications, faster onset of analgesia (39 vs 68
minutes) and far shorter lengths of stay (168 vs 267 minutes) – freeing
up beds, saving resources and allowing for more efficient management of
beds and staff time.
___________________________________
Farnia, M. R., A. Jalali, et al. (2017). "Comparison of intranasal
ketamine versus IV morphine in reducing pain in patients with renal
colic." Am J Emerg Med 35(3):
434-437.
Abstract:
BACKGROUND: Various drugs have been used to relieve abdominal
pain in patients with renal colic. Ketamine is a popular choice as an
analgesic. OBJECTIVE: To compare the effectiveness of intranasal (IN)
ketamine versus intravenous (IV) morphine in reducing pain in patients
with renal colic. METHODS: A randomized double-blind controlled trial
was performed in 53 patients with renal colic recruited from the
emergency department (ED) in 2015. Finally, 40 patients were enrolled in
this study. Patients in the ketamine group received IN ketamine 1 mg/kg
and IV placebo while patients in the control group received IV morphine
0.1mg/kg and IN placebo. Our goal was to assess visual analogue scale
(VAS) changes between the 2 groups. Patients' VAS scores were reported
before and 5, 15, 30min after drug injection. RESULTS: Before drug
administration, the mean+/-SD VAS score was 7.40+/-1.18 in the morphine
group (group A) and 8.35+/-1.30 in the ketamine group (group B)
(P-value=0.021). After adjustment by the appropriate analysis, the
mean+/-SD VAS score in group (A) and (B) at 5min were (6.07+/-0.47 vs
6.87+/-0.47; mean difference -0.79, 95% confidence interval (CI) -1.48
to -1.04) (P-value=0.025), at 15 and 30min, the mean+/-SD VAS score in
group (A) and (B) were (5.24+/-0.49 vs 5.60+/-0.49; mean difference
-0.36, 95% CI -1.08 to 0.34) and (4.02+/-0.59 vs 4.17+/-0.59; mean
difference -0.15, 95% CI -1.02 to 0.71) (P-value=0.304 and 0.719)
respectively. CONCLUSIONS: IN ketamine may be effective in decreasing
pain in renal colic.
Web site Editorial comments:
I included this article in the featured section because we need
alternatives to opiates for many painful conditions in the ED given the
ongoing opiate epidemic and the known addictive potential of these
medications. Maybe IN ketamine is a good alternative? Or maybe it could
lead to addiction as well.
Of further interest is the ability to reduce resources
if we were to treat many of our renal colic cases with IN ketamine and
either IN or buccal nausea medications.
Most of these patients need little other care – just
control of their symptoms, confirmation they have not renal infectious
process and at times renal function testing – none of which require and
IV. It is a thought for future studies: Randomize patients
to transmucosal therapy and no IV versus standard
therapy and look at cost of care, length of stay and efficacy of
treatment.
___________________________________
Mellion, S. A., D. Bourne, et al. (2017). "Evaluating Clinical
Effectiveness and Pharmacokinetic Profile of Atomized Intranasal
Midazolam in Children Undergoing Laceration Repair." J Emerg Med
53(3): 397-404.
Abstract:
BACKGROUND: Atomized intranasal midazolam is a common adjunct in pediatrics for procedural anxiolysis. There are no previous studies of validated anxiety scores with pharmacokinetic data to support optimal procedure timing. OBJECTIVES: We describe the clinical and pharmacokinetic profile of atomized intranasal midazolam in children presenting for laceration repair. METHODS: Children 11 months to 7 years of age and weighing <26 kg received 0.4 mg/kg of atomized intranasal midazolam for simple laceration repair. Blood samples were obtained at 3 time points in each patient, and the data were fit with a 1-compartment model. Patient anxiety was rated with the Observational Scale of Behavioral Distress. Secondary outcomes included use of adjunctive medications, successful completion of procedure, and adverse events. RESULTS: Sixty-two subjects were enrolled, with a mean age of 3.3 years. The median time to peak midazolam concentration was 10.1 min (interquartile range 9.7-10.8 min), and the median time to the procedure was 26 min (interquartile range 21-34 min). There was a trend in higher Observational Scale of Behavioral Distress scores during the procedure. We observed a total of 2 adverse events, 1 episode of vomiting (1.6%) and 1 paradoxical reaction (1.6%). Procedural completion was successful in 97% of patients. CONCLUSIONS: Atomized intranasal midazolam is a safe and effective anxiolytic to facilitate laceration repair. The plasma concentration was >90% of the maximum from 5 to 17 min, suggesting this as an ideal procedural timeframe after intranasal midazolam administration.
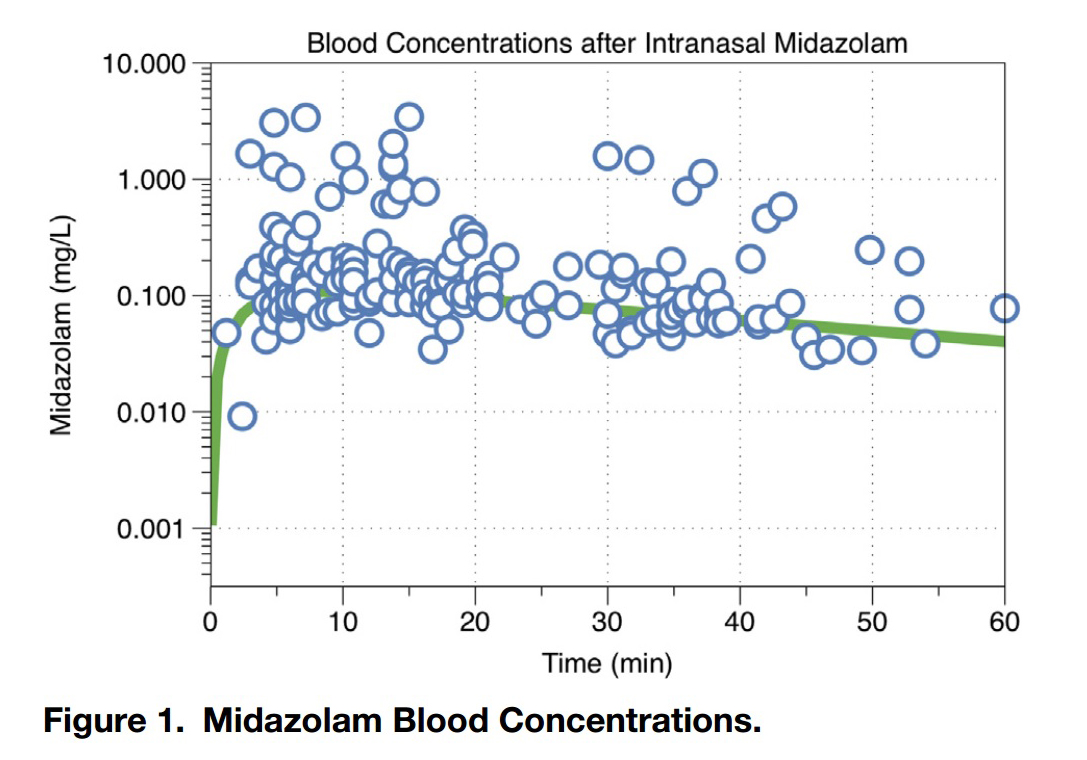
Mellion Figure 1: Midazolam blood concentration
This graph demonstrates the rapid absorption of 5 mg/ml generic
midazolam when administered by a syringe driven atomizer. The peak is in
10 minutes and the 90% of peak time range is 5 to 17 minutes at which
point it begins to drop off (this
is a logarithmic scale so the slope is not as steep looking as a
normal scale). This timing of the peak suggests the ideal time to do the
procedure (start work as soon as they are goofy which is about 5
minutes, go fast as it wears o ff relatively quickly).
Web site Editorial comments:
This study confirms in a research study what this website has recommended all along: You have limited time and must utilize it all very efficiently to successfully use nasal midazolam for laceration repair. The peak is at 10 minutes, the 90% peak time frame is 5-17 minutes and the half life is 33 minutes so it comes on quickly but goes away quickly. Here is the synopsis of their recommendations: Give the kid a nasal dose of lidocaine first then get all your tools out and ready (our recommendation); administer a weight based dose of nasal midazolam in the 0.4 to 0.5 mg/kg range (higher is better); as soon as they are goofy (about 5 minutes) position them, anesthetize and begin cleansing the wound (this requires some restraint as they are not unconscious); sew it. Do all this in 20-30 minutes if possible. If you will need longer sedation time for a complex wound consider alternate sedative like IM ketamine.
Pubmed link: https://www.ncbi.nlm.nih.gov/pubmed/28992870___________________________________
Reynolds, S. L., K. K. Bryant, et al. (2017).
"Randomized Controlled Feasibility Trial of Intranasal Ketamine Compared
to Intranasal Fentanyl for Analgesia in Children with Suspected
Extremity Fractures." Acad Emerg Med.
Abstract:
OBJECTIVE: We compared the tolerability and efficacy of intranasal
sub-dissociative ketamine to intranasal fentanyl for analgesia of
children with acute traumatic pain and investigated the feasibility of a
larger non-inferiority trial that could investigate the potential opioid
sparing effects of intranasal ketamine. METHODS: This randomized
controlled trial compared intranasal ketamine 1 mg/kg to intranasal
fentanyl 1.5 mug/kg in children 4-17 years old with acute pain from
suspected, isolated extremity fractures presenting to an urban level II
pediatric trauma center from December 2015 to November 2016. Patients,
parents, treating physicians, and outcome assessors were blinded to
group allocation. The primary outcome, a tolerability measure, was the
frequency of cumulative side effects and adverse events within 60
minutes of drug administration. The secondary outcomes included the
difference in mean pain score reduction at 20 minutes, the proportion of
patients achieving a clinically significant reduction in pain in 20
minutes, total dose of opioid pain medication in morphine
equivalents/kg/hour (excluding study drug) required during the emergency
department (ED) stay, and the feasibility of enrolling children
presenting to the ED in acute pain into a randomized trial conducted
under US regulations. All patients were monitored until 6 hours after
their last dose of study drug, or until admission to the hospital ward
or operating room. RESULTS: Of 629 patients screened, 87 received the
study drug and 82 had complete data for the primary outcome (41 patients
in each group). The median age (interquartile range) was 8 (3) years and
62% were male. Baseline pain scores were similar among patients
randomized to receive ketamine (73 +/- 26) and fentanyl (69 +/- 26)
[mean difference (95% CI): 4 (-7 to 15)]. The cumulative number of side
effects was 2.2 times higher in the ketamine group, but there were no
serious adverse events and no patients in either group required
intervention. The most common side effects of ketamine were bad taste in
the mouth (37; 90.2%), dizziness (30; 73.2%), and sleepiness (19;
46.3%). The most common side effects of fentanyl were sleepiness (15;
36.6%), bad taste in the mouth (9; 22%), and itchy nose (9; 22%). No
patients experienced respiratory side effects. At 20 minutes, the mean
pain scale score reduction was 44 +/- 36 for ketamine and 35 +/- 29 for
fentanyl [mean difference: 9 (95% CI: -4 to 23)]. Procedural sedation
with ketamine occurred in 28 ketamine patients (65%) and 25 fentanyl
patients (57%) prior to completing the study. CONCLUSIONS: Intranasal
ketamine was associated with more minor side effects than intranasal
fentanyl. Pain relief at 20 minutes was similar between groups. Our data
support the feasibility of a larger, non-inferiority trial to more
rigorously evaluate the safety, efficacy, and potential opioid sparing
benefits of intranasal ketamine analgesia for children with acute pain.
This article is protected by copyright. All rights reserved.
Web site Editorial comments:
This article is included here due to results that show we may be able to reduce opiate use safely and effectively simply by using IN ketamine for acute pain control. As in other studies they did find more side effects using ketamine, all were minor and relatively non-concerning given the issues that now surround opiates. I have been resistant to using ketamine for acute pain in the ED as I have extensive experience with IN fentanyl at 2 mcg/kg in kids and IN sufentanil at 0.5 to 0.7 mcg/kg in adults and find them almost 100% effective (titration to effect is rarely needed but easily done so you can almost always achieve very adequate pain control). However, given the opiate epidemic we are now suffering I can see it may be time to consider a change and look for other alternatives. It looks like IN ketamine may offer such an alternative and it is a single drug with a single weight based dosing regimen for both kids and adults so I am interested in possibly changing my recommendations. I might push my doses a bit higher than theirs as there is plenty of other literature demonstrating the safety of a higher ketamine dose and some enhanced efficacy.
Pubmed link: https://www.ncbi.nlm.nih.gov/pubmed/28926159___________________________________
Milesi, C., J. Baleine, et al. (2017). "Nasal midazolam vs ketamine for
neonatal intubation in the delivery room: a randomised trial." Arch
Dis Child Fetal Neonatal Ed.
Abstract:
OBJECTIVE: To compare the effectiveness of sedation by intranasal
administration of midazolam (nMDZ) or ketamine (nKTM) for neonatal
intubation. DESIGN: A multicentre, prospective, randomised, double-blind
study. SETTING: Delivery rooms at four tertiary perinatal centres in
France. PATIENTS: Preterm neonates with respiratory distress requiring
non-emergent endotracheal intubation for surfactant instillation.
INTERVENTIONS: Treatment was randomly allocated, with each neonate
receiving a bolus of 0.1 mL/kg in each nostril, corresponding to 0.2
mg/kg for nMDZ and 2 mg/kg for nKTM. The drug was repeated once 7 min
later at the same dose if adequate sedation was not obtained. MAIN
OUTCOME MEASURES: Success was defined by adequate sedation before
intubation and adequate comfort during the procedure. Intubation
features, respiratory and cardiovascular events were recorded. RESULTS:
Sixty newborns, with mean (SD) gestational age and birth weight of 28
(3) weeks and 1100 (350) g, were included within the first 20 min of
life. nMDZ was associated with a higher success rate (89% vs 58%; RR:
1.54, 95% CI 1.12 to 2.12, p<0.01) and shorter delays between the first
dose and intubation (10 (6) vs 16 (8) min, p<0.01).Number of attempts,
time to intubation, mean arterial blood pressure measures over the first
12 hours after birth and length of invasive ventilation were not
different. CONCLUSIONS: nMDZ was more efficient than nKTM to adequately
sedate neonates requiring intubation in the delivery room. The
haemodynamic and respiratory effects of both drugs were comparable.
CLINICAL TRIAL: This clinical trial was recorded on the National Library
of Medicine registry (NCT01517828).
Web site Editorial comments:
The results of this study should not surprise our readers as the data is very clear that nasal ketamine is very effective for pain control in this dose range, but a MUCH higher dose is needed for sedation. The authors found low dose ketamine at 2 mg/kg only 58% effective at sedating neonates while low dose midazolam (0.2 mg/kg) was 89% effective. We would expect ketamine at 2 mg/kg to have almost no sedative effects and in older children 0.2 mg/kg midazolam will stop seizures but give minimal sedation. However, this study is important for another reason – it is one of our few studies noting both safety and efficacy of nasal drugs in neonates who often do not have IV access readily available.
Pubmed link: https://www.ncbi.nlm.nih.gov/pubmed/28818854___________________________________
Schrier, L., R. Zuiker, et
al. (2017). "Pharmacokinetics and pharmacodynamics of a new highly
concentrated intranasal midazolam formulation for conscious sedation."
Br J Clin Pharmacol 83(4):
721-731.
Abstract:
AIM: To evaluate the pharmacokinetics, pharmacodynamics, nasal
tolerance and effects on sedation of a highly concentrated aqueous
intranasal midazolam formulation (Nazolam) and to compare these to
intravenous midazolam. METHODS: In this four-way crossover,
double-blind, double-dummy, randomized, placebo-controlled study, 16
subjects received 2.5 mg Nazolam, 5.0 mg Nazolam, 2.5 mg intravenous
midazolam or placebo on different occasions. Pharmacokinetics of
midazolam and alpha-hydroxy-midazolam were characterized and related to
outcome variables for sedation (saccadic peak velocity, the Bond and
Lader visual analogue scale for sedation, the simple reaction time task
and the observer's assessment of alertness/sedation). Nasal tolerance
was evaluated through subject reporting, and ear, nose and throat
examination. RESULTS: Nazolam bioavailability was 75%. Maximal plasma
concentrations of 31 ng ml-1 (CV, 42.3%) were reached after 11 min (2.5
mg Nazolam), and of 66 ng ml-1 (coefficient of variability, 31.5%) after
14 min (5.0 mg Nazolam). Nazolam displayed a significant effect on OAA/S
scores. Sedation onset (based on SPV change) occurred 1 +/- 0.7 min
after administration of 2.5 mg intravenous midazolam, 7 +/- 4.4 min
after 2.5 mg Nazolam, and 4 +/- 1.8 min after 5 mg Nazolam. Sedation
duration was 118 +/- 95.6 min for 2.5 mg intravenous midazolam, 76 +/-
80.4 min for 2.5 mg Nazolam, and 145 +/- 104.9 min for 5.0 mg Nazolam.
Nazolam did not lead to nasal mucosa damage. CONCLUSIONS: This study
demonstrates the nasal tolerance, safety and efficacy of Nazolam. When
considering the preparation time needed for obtaining venous access,
conscious sedation can be achieved in the same time span as needed for
intravenous midazolam. Nazolam may offer important advantages in
conscious sedation.
Web site Editorial comments:
This study reports pharmacokinetic data related to a new highly
concentrated from of Midazolam designed for IN delivery called Nazolam.
It has 75% bioavailability, fairly rapid onset and good duration of
action and does not damage mucosa.
We can expect this to be on the market soon. It is a very well designed
product that will be prepackaged as single dose applicators making them
very convenient to administer and highly predictable in their effects.
If priced and marketed correctly these products should replace our
current techniques of using generic drugs. The question of course will
be affordability when compared to the less concentrated inexpensive
generic concentrations. Hopefully the manufacturers will find a middle
ground price structure where they make a fair profit and the price is
affordable so that widespread use occurs.
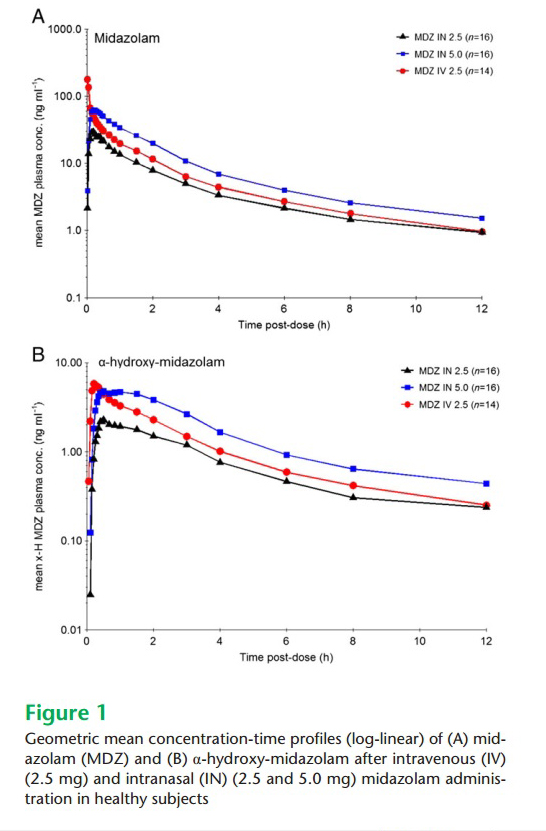
Schrier - Graph of midazolam concentration versus time profiles: This
graph demonstrates two important findings: First the high immediate peak
for IV midazolam but the lack of an immediate high peak concentration
for nasal midazolam – meaning the risk of respiratory depression is
minimal using the nasal drug. Second, nearly equivalent plasma levels of
nasal and IV drugs once the high risk high peak levels abate – meaning
similar sedative and anti-seizure effects regardless of which route of
delivery is chosen.
___________________________________
Smith, D., H. Cheek, et al. (2017).
"Lidocaine Pretreatment Reduces the Discomfort of Intranasal Midazolam
Administration: A Randomized, Double-blind, Placebo-controlled Trial."
Acad Emerg Med 24(2):
161-167.
Abstract:
OBJECTIVE: Intranasal (IN) midazolam is a commonly prescribed medication for pediatric sedation and anxiolysis. One of its most frequently encountered adverse effects is discomfort with administration. While it has been proposed that premedicating with lidocaine reduces this undesirable consequence, this combination has not been thoroughly researched. The objective of our study was to assess whether topical lidocaine lessens the discomfort associated with IN midazolam administration. METHODS: This was a double-blind, randomized, placebo-controlled trial performed in an urban, academic pediatric emergency department. Children 6-12 years of age who were receiving IN midazolam for procedural sedation received either 4% lidocaine or 0.9% saline (placebo) via mucosal atomizer. Subjects were subsequently given IN midazolam in a similar fashion and then rated their discomfort using the Wong-Baker FACES Pain Rating Scale (WBS). The primary endpoint of WBS score was analyzed with a two-tailed Mann-Whitney U-test, with p < 0.05 considered statistically significant. RESULTS: Seventy-seven patients were enrolled over a consecutive 8-month period. One child was excluded from analysis due to a discrepancy in recording the drug identification number. Study groups were similar in regard to demographic information and indication for sedation. Subjects who received IN lidocaine reported less discomfort with IN midazolam administration (median WBS = 3, interquartile range [IQR] = 0-6) than those who received placebo (median WBS = 8, IQR = 2-9; p = 0.006). CONCLUSIONS: Premedication with topical lidocaine reduces the discomfort associated with administration of IN midazolam (ClinicalTrials.gov, NCT02396537).
Web site Editorial comments:
Here is another study in a list of similar studies that show
pre-administration of lidocaine will significantly decrease the pain
children experience when they are administered IN midazolam. This study
is the most rigorous yet and shows and impressive difference in
discomfort between those treated with lidocaine and those given placebo
5 minutes prior to midazolam administration. This pretreatment should be
added to all sedation protocols using nasal midazolam administration.
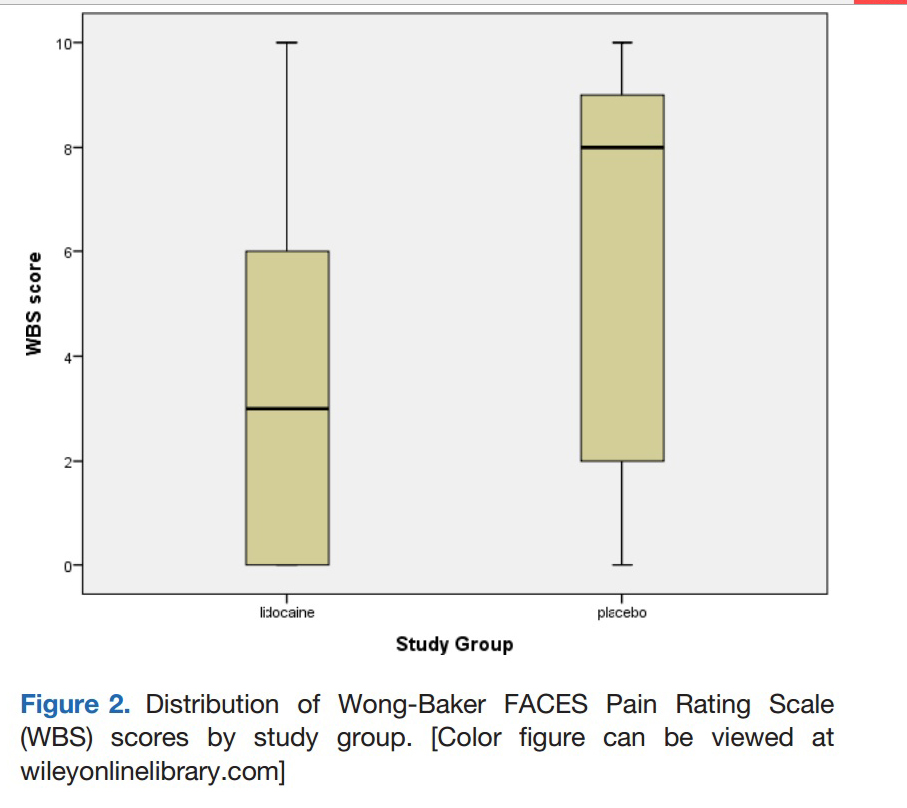
Smith et al: Graph contrasting pain scores of patients who received
lidocaine (WBS = 3) versus placebo (WBS = 8) 5 minutes prior to IN
midazolam administration.
___________________________________
Sanchez Fernandez, I., M. Gainza-Lein, et
al. (2017). "Nonintravenous rescue medications for pediatric status
epilepticus: A cost-effectiveness analysis." Epilepsia
58(8): 1349-1359.
Abstract:
OBJECTIVE: To quantify the cost-effectiveness of rescue medications for
pediatric status epilepticus: rectal diazepam, nasal midazolam, buccal
midazolam, intramuscular midazolam, and nasal lorazepam. METHODS:
Decision analysis model populated with effectiveness data from the
literature and cost data from publicly available market prices. The
primary outcome was cost per seizure stopped ($/SS). One-way sensitivity
analyses and second-order Monte Carlo simulations evaluated the
robustness of the results across wide variations of the input
parameters. RESULTS: The most cost-effective rescue medication was
buccal midazolam (incremental cost-effectiveness ratio ([ICER]:
$13.16/SS) followed by nasal midazolam (ICER: $38.19/SS). Nasal
lorazepam (ICER: -$3.8/SS), intramuscular midazolam (ICER: -$64/SS), and
rectal diazepam (ICER: -$2,246.21/SS) are never more cost-effective than
the other options at any willingness to pay. One-way sensitivity
analysis showed the following: (1) at its current effectiveness, rectal
diazepam would become the most cost-effective option only if its cost
was $6 or less, and (2) at its current cost, rectal diazepam would
become the most cost-effective option only if effectiveness was higher
than 0.89 (and only with very high willingness to pay of $2,859/SS to
$31,447/SS). Second-order Monte Carlo simulations showed the following:
(1) nasal midazolam and intramuscular midazolam were the more effective
options; (2) the more cost-effective option was buccal midazolam for a
willingness to pay from $14/SS to $41/SS and nasal midazolam for a
willingness to pay above $41/SS; (3) cost-effectiveness overlapped for
buccal midazolam, nasal lorazepam, intramuscular midazolam, and nasal
midazolam; and (4) rectal diazepam was not cost-effective at any
willingness to pay, and this conclusion remained extremely robust to
wide variations of the input parameters. SIGNIFICANCE: For pediatric
status epilepticus, buccal midazolam and nasal midazolam are the most
cost-effective nonintravenous rescue medications in the United States.
Rectal diazepam is not a cost-effective alternative, and this conclusion
remains extremely robust to wide variations of the input parameters.
Web site Editorial comments:
This article confirms what all of us in practice know – rectal
diazepam is not as effective as nasal or buccal drugs and it costs an
exorbitant amount of money ($326/dose versus $6 to $12/dose for buccal
and nasal midazolam) for its poor performance so it is not worth using
given all our better options. The only reason this author thinks it can
possibly still be in active use is it has regulatory approval for
treating increasingly frequent seizures, while nasal and buccal drugs
are generic IV formulations without regulator approval for other modes
of delivery. Interestingly, they point out that rectal diazepam does NOT
have regulatory approval for treating ongoing seizures or status
epilepticus so it is in effect being used off label as well. (It
is approved just
for reducing frequency of seizures.)
It is unfortunate
that today, despite the fairly compelling evidence of superior efficacy
(and outcomes) we clinicians still prescribe this outdated rectal drug
and force our patients to pay its ridiculous costs for worse results and
a socially unacceptable delivery method.
These authors and I
predict it will disappear as soon as nasal benzodiazepines cross the
regulatory approval threshold. They point out that rectal diazepam NOT
recommended for use in status epilepsy by the American epilepsy society
– rather they recommend non-IV midazolam delivery methods.
I also predict that buccal and IM formulations will
struggle to compete with the nasal drug once it gets regulatory approval
as there is a fair amount of literature showing more rapid onset of
seizure control and slightly higher efficacy using the nasal
formulations.
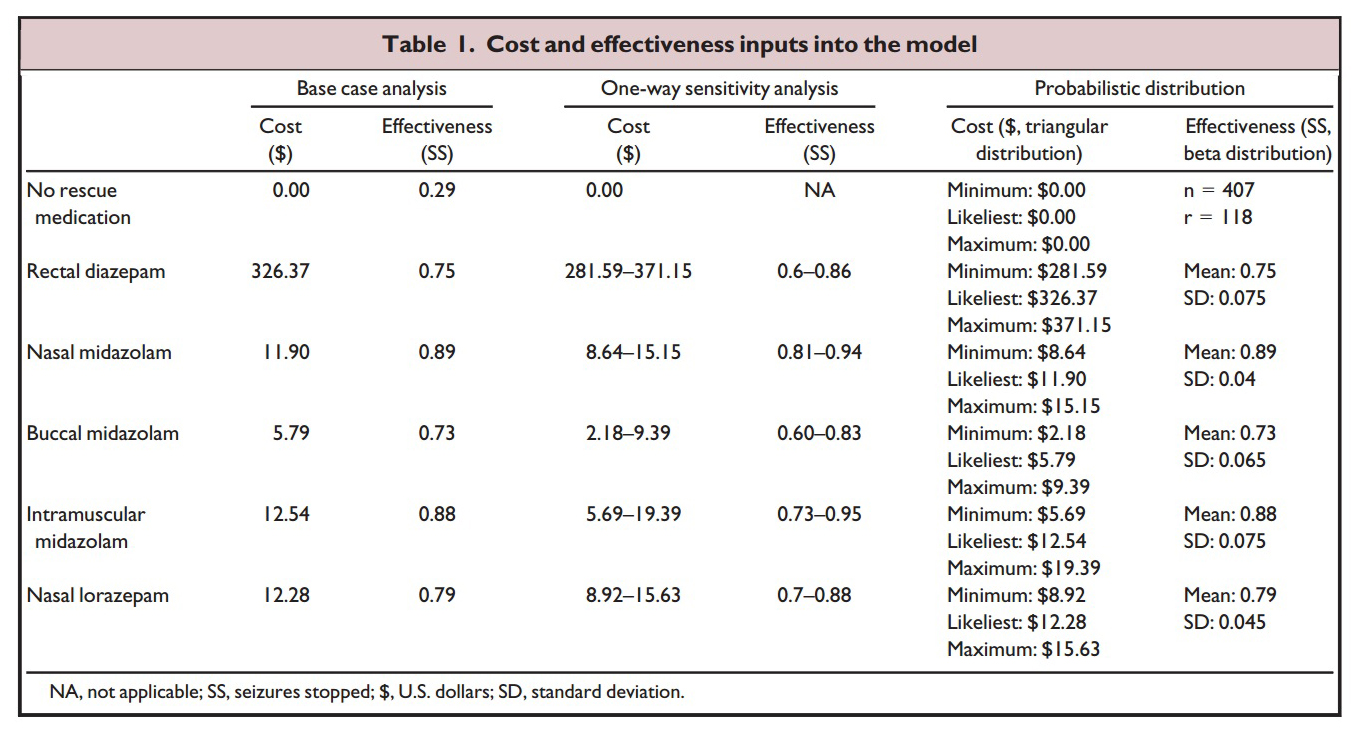
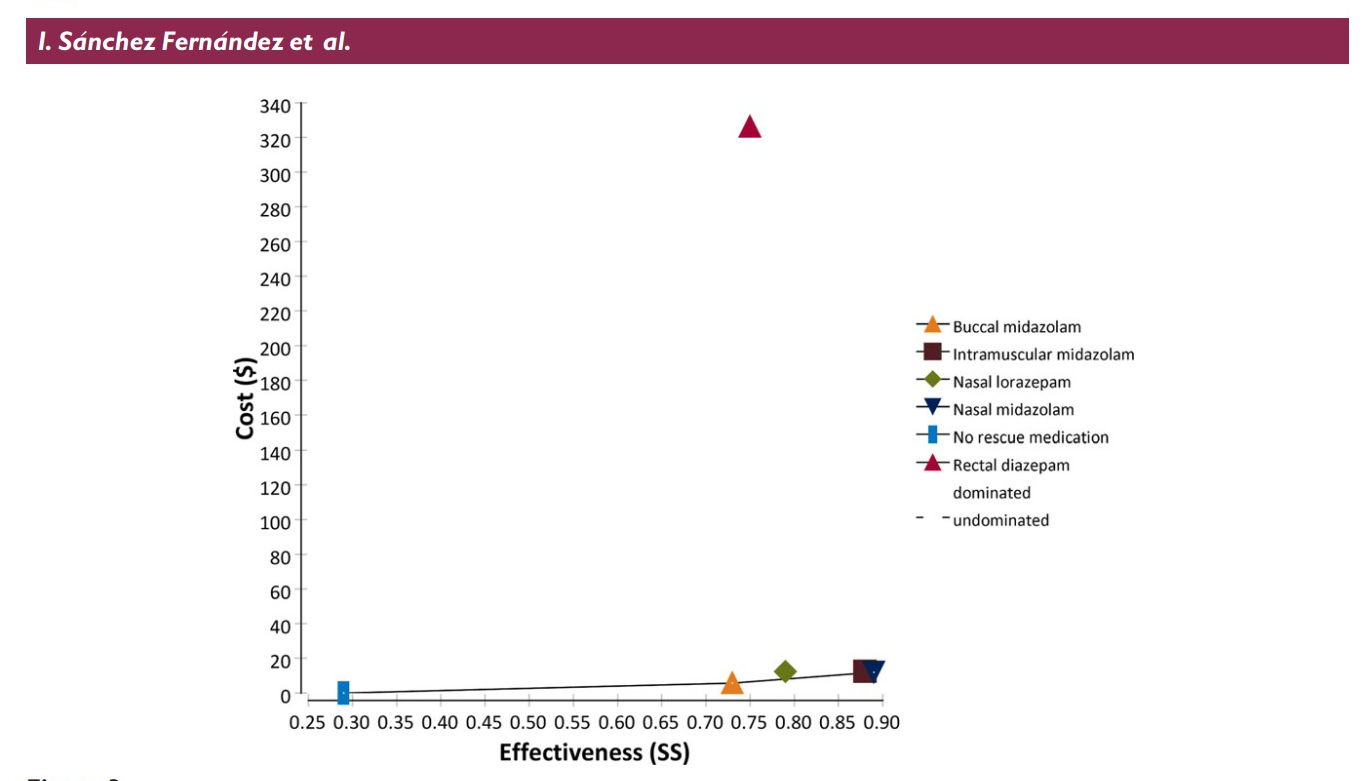
The table and graph above shows the parameters Sanchez Fernandez et al
used to determine their results: Cost is the total cost of the drug plus
applicator (nasal atomizer or syringe and needle etc). Effectiveness is
the literature reported percentage of cases (from 24 studies reviewed)
that the medication and delivery route will stop prolonged seizures. The
graph plots the cost versus the effectiveness with the ideal drug being
one that plots out at the lower right corner of the graph (low cost,
highly effective). As is readily apparent rectal diazepam is an absolute
outlier.
___________________________________
Bailey, A. M., R. A. Baum, et al. (2017). "Review of Intranasally Administered Medications for Use in the Emergency Department." J Emerg Med 53(1): 38-48
McBride, D. L. (2017). "Emergency Departments Increasingly Administering
Medications through the Nose." J Pediatr Nurs.
Rech,
M. A., B. Barbas, et al. (2017). "When to Pick the Nose: Out-of-Hospital
and Emergency Department Intranasal Administration of Medications."
Ann Emerg Med 70(2):
203-211.
Abstracts:
BACKGROUND: Intranasal (IN) medication delivery is a viable
alternative to other routes of administration, including intravenous
(IV) and intramuscular (IM) administration. The IN route bypasses the
risk of needle-stick injuries and alleviates the emotional trauma that
may arise from the insertion of an IV catheter. OBJECTIVE: This review
aims to evaluate published literature on medications administered via
the IN route that are applicable to practice in emergency medicine.
DISCUSSION: The nasal mucosa is highly vascularized, and the olfactory
tissues provide a direct conduit to the central nervous system, bypass
first-pass metabolism, and lead to an onset of action similar to IV drug
administration. This route of administration has also been shown to
decrease delays in drug administration, which can have a profound impact
in a variety of emergent scenarios, such as seizures, acutely agitated
or combative patients, and trauma management. IN administration of
midazolam, lorazepam, flumazenil, dexmedetomidine, ketamine, fentanyl,
hydromorphone, butorphanol, naloxone, insulin, and haloperidol has been
shown to be a safe, effective alternative to IM or IV administration. As
the use of IN medications becomes a more common route of administration
in the emergency department setting, and in prehospital and outpatient
settings, it is increasingly important for providers to become more
familiar with the nuances of this novel route of medication delivery.
CONCLUSIONS: IN administration of the reviewed medications has been
shown to be a safe and effective alternative to IM or IV administration.
Use of IN is becoming more commonplace in the emergency department
setting and in prehospital settings.
Web site Editorial comments:
These three articles by authors Bailey, McBride and Rech review the literature regarding the use of IN drugs in the emergency department. They don't really fit anywhere on this site as they are review articles that essentially confirm what is written here on this web site, citing some of the many articles within these pages. Their publication made me feel good as it now appears that in 2017 the concept of IN drug delivery is finally going mainstream in US emergency medicine literature (it was adopted by many ED clinicians far earlier: 1- 2 decades ago).
Pubmed links:https://www.ncbi.nlm.nih.gov/pubmed/28259526
https://www.ncbi.nlm.nih.gov/pubmed/28551041
https://www.ncbi.nlm.nih.gov/pubmed/28366351
___________________________________
Nemeth, M., N. Jacobsen, et al. (2017). "Intranasal Analgesia and
Sedation in Pediatric Emergency Care-A Prospective Observational Study
on the Implementation of an Institutional Protocol in a Tertiary
Children's Hospital." Pediatr Emerg Care.
Abstract:
OBJECTIVES: Children presenting with acute traumatic pain or in
need of therapeutic or diagnostic procedures require rapid and effective
analgesia and/or sedation. Intranasal administration (INA) promises to
be a reliable, minimally invasive delivery route. However, INA is still
underused in Germany. We hence developed a protocol for acute pain
therapy (APT) and urgent analgesia and/or sedation (UAS). Our aim was to
evaluate the effectiveness and safety of our protocol. METHODS: We
performed a prospective observational study in a tertiary children's
hospital in Germany. Pediatric patients aged 0 to 17 years requiring APT
or UAS were included. Fentanyl, s-ketamine, midazolam, or combinations
were delivered according to protocol. Primary outcome variables included
quality of analgesia and/or sedation as measured on age-appropriate
scales and time to onset of drug action. Secondary outcomes were adverse
events and serious adverse events. RESULTS: One hundred pediatric
patients aged 0.3 to 16 years were enrolled, 34 for APT and 66 for UAS.
The median time onset of drug action was 5 minutes (ranging from 2 to 15
minutes). Fentanyl was most frequently used for APT (n = 19). Pain
scores decreased by a median of 4 points (range, 0-10; P < 0.0001). For
UAS, s-ketamine/midazolam was most frequently used (n = 25). Sedation
score indicated minimal sedation in most cases. Overall success rate
after the first attempt was 82%. Adverse events consisted of nasal
burning (n = 2) and vomiting (n = 2). No serious adverse events were
recorded. CONCLUSIONS: A fentanyl-, s-ketamine-, and midazolam-based INA
protocol was effective and safe for APT and UAS. It should then be
considered where intravenous access is impossible or inappropriate.
Web site Editorial comments:
This study is one of the few nasal drug delivery papers coming out of Germany. As these authors elude, German physicians have been a bit hesitant to adopt this treatment modality until they see more evidence from their own colleagues. Here is a very well done study using adequate doses of IN drugs and an atomizer to deliver the drugs – so the results should be reliable and believable. The authors provide good evidence that IN drug delivery is safe and effective for sedation and pain control when delivered by German clinicians to their patients using the protocols and doses they recommend.
Pubmed link: https://www.ncbi.nlm.nih.gov/pubmed/28121974___________________________________
Tsze, D. S., M. Ieni, et
al. (2017). "Optimal Volume of Administration of Intranasal Midazolam in
Children: A Randomized Clinical Trial." Ann Emerg Med
69(5): 600-609.
Abstract:
STUDY OBJECTIVE: The optimal intranasal volume of administration for
achieving timely and effective sedation in children is unclear. We aimed
to compare clinical outcomes relevant to procedural sedation associated
with using escalating volumes of administration to administer intranasal
midazolam. METHODS: We conducted a randomized, single-blinded, 3-arm,
superiority clinical trial. Children aged 1 to 7 years and undergoing
laceration repair requiring 0.5 mg/kg intranasal midazolam (5 mg/mL)
were block-randomized to receive midazolam using 1 of 3 volumes of
administration: 0.2, 0.5, or 1 mL. Procedures were videotaped, with
outcome assessors blinded to volume of administration. Primary outcome
was time to onset of minimal sedation (ie, score of 1 on the University
of Michigan Sedation Scale). Secondary outcomes included procedural
distress, time to procedure start, deepest level of sedation achieved,
adverse events, and clinician and caregiver satisfaction. RESULTS:
Ninety-nine children were enrolled; 96 were analyzed for the primary
outcome and secondary outcomes, except for the outcome of procedural
distress, for which only 90 were analyzed. Time to onset of minimal
sedation for each escalating volume of administration was 4.7 minutes
(95% confidence interval [CI] 3.8 to 5.4 minutes), 4.3 minutes (95% CI
3.9 to 4.9 minutes), and 5.2 minutes (95% CI 4.6 to 7.0 minutes),
respectively. There were no differences in secondary outcomes except for
clinician satisfaction with ease of administration: fewer clinicians
were satisfied when using a volume of administration of 0.2 mL.
CONCLUSION: There was a slightly shorter time to onset of minimal
sedation when a volume of administration of 0.5 mL was used compared
with 1 mL, but all 3 volumes of administration produced comparable
clinical outcomes. Fewer clinicians were satisfied with ease of
administration with a volume of administration of 0.2 mL.
Web site Editorial comments:
This is an important clinical article for those who are interested
in a deeper understanding of intranasal medication delivery and the
pharmaceutical/FDA approach to dosing and concentration versus the
approach of clinicians who just want to help their patients. In order
for a pharmaceutical company to get a nasal medication approved they
need to achieve certain parameters set down by the FDA or other
regulatory body. One of those parameters is often a bioavailability test
of the nasal drug when compared to an already approved method of
delivering the same medication such as via the intramuscular route. For
example, the FDA required that IN
naloxone bioavailability needed to be the same as IM naloxone before
they would approve the commercially available kit released in 2015. This
bioavailability was determined to be 40% compared to the same dose give
IV (which for all intents and purposes is 100% bioavailable). To achieve
this bioavailability there can be NO waste of drug due to run-off
(regardless of whether that waste allows more total drug to be absorbed
by giving a higher mg dose). For this reason nasal drugs are highly
concentrated and packaged into volumes of around 0.1 ml for delivery.
While this small volume when delivered in a volunteer setting ensures no
run-off and high bioavailability, it is not necessarily optimal for
clinical use. Clinicians in the real world want the best clinical effect
which is not the same as the best bioavailability. Tiny doses (0.1 ml)
are hard to deliver and might accidentally be sprayed onto the nasal ala
or septum with little resulting clinical efficacy. Volumes in the 0.3 to
0.5 range are clinically easier to deliver, cover a larger surface area
and a little waste is fine as long as the clinical effect is adequate
and no additional side effects occur. This study confirms this concept
in the real world and finds that the 0.5 ml
or 1 ml volume
is easier to use and
preferred by clinicians to 0.2ml volume. However, its findings are
debatable because they did not give a single dose of 0.2, 0.5 or 1 ml
with variable concentrations. Instead they
gave the same weight
based volume of the drug to each child but varied the number of
administrations using 0.2, 0.5 or 1.0 ml aliquots.
Perhaps a future
study will be able to concentrate the IN medication into multiple
concentrations (similar to how diamorphine is administered – starting
with lyophylized powder and solubilizing it with three different volumes
having identical drug doses, then testing that method.)
___________________________________
Title:
Abstract:
Web site Editorial comments:
Pubmed link:
___________________________________
Weiner, S. G., P. M. Mitchell, et al. (2017). "Use of Intranasal
Naloxone by Basic Life Support Providers." Prehosp Emerg Care
21(3): 322-326.
Abstract:
STUDY OBJECTIVES: Intranasal delivery of naloxone to reverse the effects of opioid overdose by Advanced Life Support (ALS) providers has been studied in several prehospital settings. In 2006, in response to the increase in opioid-related overdoses, a special waiver from the state allowed administration of intranasal naloxone by Basic Life Support (BLS) providers in our city. This study aimed to determine: 1) if patients who received a 2-mg dose of nasal naloxone administered by BLS required repeat dosing while in the emergency department (ED), and 2) the disposition of these patients. METHODS: This was a retrospective review of patients transported by an inner-city municipal ambulance service to one of three academic medical centers. We included patients aged 18 and older that were transported by ambulance between 1/1/2006 and 12/12/2012 and who received intranasal naloxone by BLS providers as per a state approved protocol. Site investigators matched EMS run data to patients from each hospital's EMR and performed a chart review to confirm that the patient was correctly identified and to record the outcomes of interest. Descriptive statistics were then generated. RESULTS: A total of 793 patients received nasal naloxone by BLS and were transported to three hospitals. ALS intervened and transported 116 (14.6%) patients, and 11 (1.4%) were intubated in the field. There were 724 (91.3%) patients successfully matched to an ED chart. Hospital A received 336 (46.4%) patients, Hospital B received 210 (29.0%) patients, and Hospital C received 178 (24.6%) patients. Mean age was 36.2 (SD 10.5) years and 522 (72.1%) were male; 702 (97.1%) were reported to have abused heroin while 21 (2.9%) used other opioids. Nasal naloxone had an effect per the prehospital record in 689 (95.2%) patients. An additional naloxone dose was given in the ED to 64 (8.8%) patients. ED dispositions were: 507 (70.0%) discharged, 105 (14.5%) admitted, and 112 (15.5%) other (e.g., left against medical advice, left without being seen, or transferred). CONCLUSIONS: Only a small percentage of patients receiving prehospital administration of nasal naloxone by BLS providers required additional doses of naloxone in the ED and the majority of patients were discharged.
Web site Editorial comments:
This is an important study. These patients were resuscitated by BLS administration of generic low concentration naloxone (not the new highly concentrated commercially available form). The success of resuscitation was 95% - this shows that if left alone without ALS intervention, patients given generic nasal naloxone (which is probably a bit dilute) is effective in the same percentage of patients as the more expensive highly concentrated formulations or even IV naloxone. If you read some of the early studies such as the very first studies on this topic the efficacy was deemed to be around 85%, but if you dig into the methods of these early studies they were all ALS delivered drug and the ALS providers moved immediately on to further interventions including IV naloxone if they did not detect rapid effect of the IN formulation. Often, the IN drug was only in the system a few minutes before IV naloxone was given and that patient would be deemed an IN failure. This study does not have that “flaw” – it looks at actual clinical response over a longer time period and finds that 19 of 20 opioid toxic patients will respond to nasal naloxone even in the dilute 1 mg/ml formulations. So be patient, assist ventilation, let the drug do its job and if your budget is limited generic naloxone therapy is more cost effective and just as clinically effective in the hands of BLS providers as the new potentially more expensive commercially available kit. If there is no price difference, the new kits make more sense, but if there is a substantial difference in cost, this study defends using the original generic formulations.
Pubmed link: https://www.ncbi.nlm.nih.gov/pubmed/28166446
 Therapeutic
Intranasal Drug Delivery
Therapeutic
Intranasal Drug Delivery