Featured new articles related to intranasal drug delivery:
April - June 2011
Carpuject and PharMEDium concept (not an article - just a recently noted idea very pertinent to this web site topic)
Web site editorial comments
One of the potential downsides to seeing intranasal drug delivery achieve its real therapeutic potential (use by lay public and BLS provides in emergencies such as seizures and opiate overdose) has been the need to draw up the proper dose of a drug into a syringe. Prepackaged, prefilled syringes of drug would solve much of this problem. This web site provides detailed information relating to prepackaged, prefilled 2 mg syringes of naloxone for use by the lay public. Naloxone packaged in this fashion allows the user to simply connect an atomizer to the prefilled syringe and deliver the drug intranasally - simple and safe. It appears the same can be done for most of the medications we discuss on this web site. It has been recently pointed out by a viewer that commonly used IN drugs (fentanyl and midazolam) as well as many other drugs that are nasally bioavailable (lorazepam, ketorolac, hydromorphone, etc) are currently available in prefilled syringes. Based on extensive research and community use, the prepackaged midazolam would certainly seem appropriate for use by properly trained families and BLS providers - and this packaging would surely help improve the ease of use. See this PDF file for the information on these prepackaged medications: Carpuject medications; PharMEDium medications
___________________________________
Borland, M., S. Milsom, et al. (2011). "Equivalency of two concentrations of fentanyl administered by the intranasal route for acute analgesia in children in a paediatric emergency department: A randomized controlled trial." Emerg Med Australas 23(2): 202-208.
Abstract: Objective: Intranasal fentanyl's (INF) effectiveness is established using highly concentrated INF (HINF). Standard concentration INF (SINF) is more widely available. We aimed to illustrate the equivalence of SINF to HINF. Methods: Double-blinded randomized controlled trial was used within a children's hospital ED. Children aged 3-15 years with fractures were randomized to SINF or HINF. Outcome measures included pain scores at time zero and every 10 min until 30 min. Additional analgesic agents were noted. Results: Data in 189 children (91 HINF, 98 SINF) were obtained. Pre-analgesia median VAS was 80.0 mm (interquartile range [IQR] 60.0-95.5) in SINF, 77.5 mm (IQR 60.0-100) in HINF. At 10 min median VAS was 49.5 mm (IQR 26.5-68.5) and 43.0 mm (IQR 15.2-66.0), respectively, at 20 min 27.5 mm (IQR 18.5-56.5) and 35.0 mm (IQR 9.0-57.0) and at 30 min 20.0 mm (IQR 10.0-46.0) and 21.5 mm (IQR 4.75-51.0). Each agent demonstrated significant decrease in pain scores (median decrease 40 mm, P = 0.000). Additional analgesia was given in 67 (42 SINF, 25 HINF) (P = 0.028). The decrease in pain scores between children < and >/=50 kg in SINF was significant both overall (P = 0.005) and between 10 and 20 min (P = 0.003). There was no difference in HINF at any time by weight. Conclusions: The two concentrations of INF were equivalent in reducing pain, with a trend to increased oral additional agents in the more dilute solution. The widespread use of this readily available analgesic in the standard concentration can be supported, particularly in patients <50 kg.
Web site Editorial comments:
This is a great study validating current practice for most of us - just use inexpensive generic fentanyl straight out of the pharmacy to treat pain in children. While it would nice to have more concentrated fentanyl for patients over 50 kg due to volume issues, it is very easy to just use sufentanil at that point since it is also generic, inexpensive and readily available yet is 5-8 times more potent than fentanyl - in essence providing us with a ready made concentrated nasal opioid for adults and children (more recent research on the topic of IN sufentanil is coming soon and a big review on sufentanil in kids was just published and is reviewed below).
Pubmed link: http://www.ncbi.nlm.nih.gov/pubmed/21489168?dopt=Citation
___________________________________
Lundeberg, S. and J. A. Roelofse (2011). "Aspects of pharmacokinetics and pharmacodynamics of sufentanil in pediatric practice." Paediatr Anaesth 21(3): 274-279.
Abstract: Sufentanil is a potent synthetic opioid. Like other opioids, sufentanil creates a stable hemodynamic environment in cardiovascularly compromised pediatric patients. Clearance, expressed as per kilogram, is increased in children compared to adults. The P450 CYP3A4 enzyme is responsible for the major metabolic N-dealkylation pathway. Enzyme activity is reduced in neonates but the maturation of sufentanil clearance is not described. The free active fraction is affected by age because of the reduced alpha(1) -acid glycoprotein plasma concentrations in neonates. Intranasal administration of sufentanil is a possible option for premedication, procedural sedation and analgesia in children, as this option has been found to be safe and effective. Studies concerning the pharmacokinetics and dynamics of sufentanil administered as a bolus or continuous infusion in children are few.
Web site Editorial comments:
This is a very interesting and insightful article for anyone who wishes to use intranasal sufentanil in their practice. They also provide some comments on personal experience using intranasal sufentanil in over 1000 children undergoing surgery - commenting on its safety and efficacy.
Pubmed link: http://www.ncbi.nlm.nih.gov/pubmed/20849451?dopt=Citation
___________________________________
Prommer, E. and L. Thompson (2011). "Intranasal Fentanyl for pain control: current status with a focus on patient considerations." Patient Preference and Adherence (open access) 5: 157-164.
Abstract: Of several newer delivery systems under development and investigation for the administration of opioids, the intranasal route has received a substantial amount of attention. Intranasal administration is a convenient form of delivery that is applicable to several opioids. It has the potential for self-administration, combined with a rapid onset of action, allowing for patient-controlled analgesia. In clinical practice, intranasal administration has been found to be a reliable drug delivery method that is familiar to patients. Intranasal opioids have proven to be useful in both in-hospital and out-of-hospital pain management settings. Fentanyl, a highly lipophilic step 3 opioid, has been evaluated for intranasal administration. The purpose of this review is to examine the role of the nasal route of opioid administration and examine the evidence base for the use of fentanyl intranasally.
Web site Editorial comments:
This is a fairly up to date review on the topic of IN fentanyl with a primary focus towards hospice but inclusion of EMS and ER uses as well. It is an open access (free) article so can be downloaded and quickly read. Click below for the download
Free Open access article (click here)
___________________________________
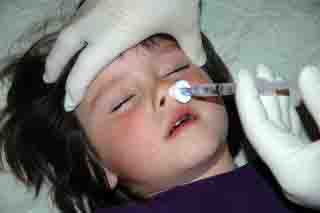 Therapeutic
Intranasal Drug Delivery
Therapeutic
Intranasal Drug Delivery